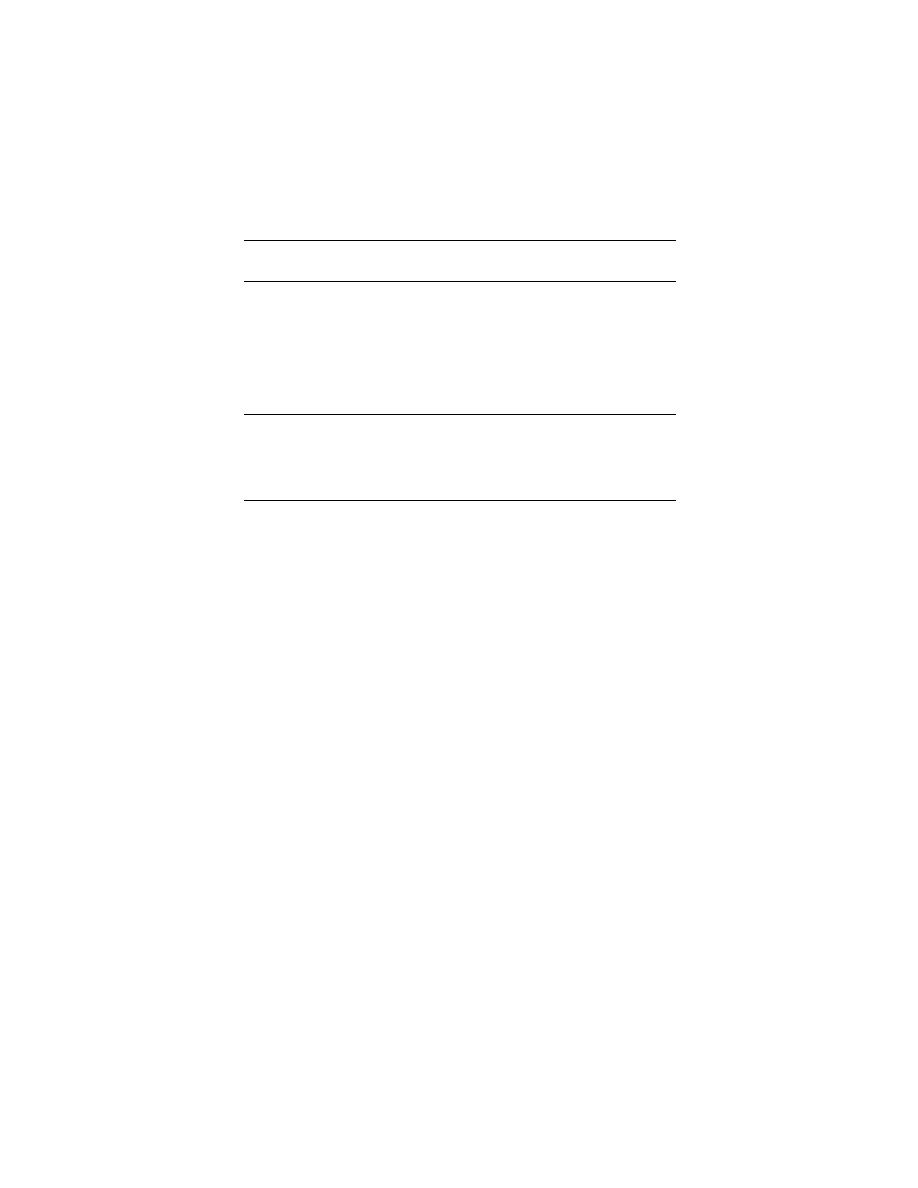
Table 3. Performance of insulated probes.
Initial tests, 26 September 1990. No attempt was made to quantify the actual
amount of salt (sodium chloride) added; these tests were run merely to demon-
strate that uninsulated probes would not perform well in saline solutions and
that insulated probes held some possibilities in this area. Tabular values are the
calculated dielectric constant for the tap water used in these tests. The insulation
on the test probe was a polyolefin heat-shrink tubing.
Distilled
Salt
More salt
More salt
water
added
added
added
Uninsulated
65.9
36.2
18.7
42.0
Insulated
52.0
56.1
59.2
65.0
Detailed tests, 16 October 1991. Epoxy resin and heat-shrink tubing used as
insulations. All readings were taken with box 2, probe circuit 1.
Probes M and U--epoxy insulation
Probes F and H--uninsulated
Probe T--insulated with heat-shrink tubing
Distilled
Probe
water
0.001 N
0.005 N
0.01 N
0.06 N
0.1 N
M
80.0
79.8
90.5
117.2
210.0
220.1
U
79.1
77.9
89.8
118.2
202.4
210.4
F
80.0
69.2
3.9
40.1
115.6
122.8
H
80.0
70.1
4.4
39.7
115.6
122.8
T
80.0
ND
ND
97.1
ND
102.6
Notes:
The dielectric constant was calculated by finding Cair for the distilled water
and then using this value to calculate the dielectric constants for the other
molarities. Potassium chloride was the salt used for these tests. Tabular values
are dielectric constants.
The uninsulated probes are essentially useless once the molarity is over 0.001 N.
The epoxy probes have an excessive error above 0.005 N.
The heat-shrink tubing is probably usable with acceptable error limits up to
about 0.01 N.
ND = no data taken at that molarity.
Box 2: Average K = 41.2
may be met using either of two methods for cali-
Box 3: Average K = 43.3
brating an insulated probe:
Box 4: Average K = 41.6.
1. Calibrate the insulated probe using water
as a reference material and apply the exact
Since the methanol has an actual value of 32.65,
same procedure as is used for an uninsulated
the results of this particular method indicate a
probe, or
rather poor accuracy for dielectric constant calcu-
2. Calculate an equivalent capacitance for the
lations.
insulation and use it as a series capacitance
Applying procedure 2, the calculated values
to correct the insulated probe readings to
for methanol are shown in Table 5. The results of
indicate the same capacitance as an unin-
this method for calculating the dielectric constant
sulated probe.
are summarized below:
Using procedure 1, the calculated values for
Box 2: Average K = 29.3
methanol are shown in Table 4. The results of this
Box 3: Average K = 31.2
method for calculating the dielectric constant of a
Box 4: Average K = 28.1
known material are summarized here:
9




 Previous Page
Previous Page
