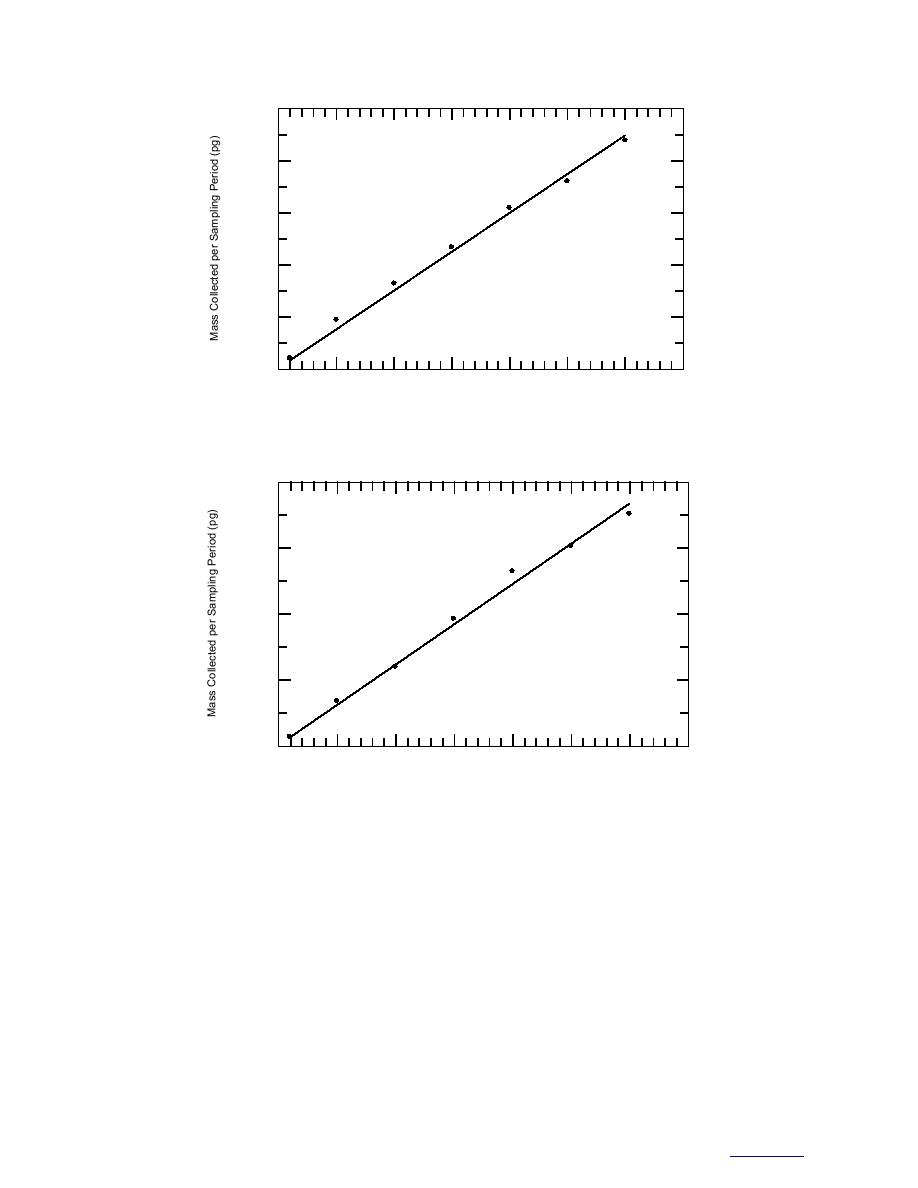
500,000
^
y = 14,900x
2
r = 0.992
400,000
300,000
200,000
100,000
0
5
10
15
20
25
30
0
35
Sorption Time (min)
Figure 10. Mass of 2,6-DNT vapor sorbed on a polyacrylate SPME fiber at 23C.
400,000
300,000
^
y = 12,200x
2
r = 0.992
200,000
100,000
0
0
35
5
10
15
20
25
30
Sorption Time (min)
Figure 11. Mass of 1,3-DNB vapor sorbed on a polyacrylate SPME fiber at 23C.
nitro groups oriented meta to each other, it is not
2.3-mL/min sampling volume we obtained for
unexpected that sampling volumes, probably con-
2,4,6-TNT, but is only about one-half of that
trolled by the rate of diffusion in the polyacrylate
obtained for the two isomers of DNT. The estimate
fiber, would be quite similar. In addition, the
of vapor pressure for this compound was
vapor pressure estimates used to estimate these vol-
obtained by extrapolation of data at higher tem-
umes were obtained from the same source (Pella
peratures and we do not feel that this value is as
1977).
reliable as the vapor pressure data for 2,4- and 2,6-
When we obtained an estimate of the effective
DNT.
sampling volume for 1,3-DNB from the data
Effective sampling rates were obtained for 2,4,6-
shown in Figure 11, however, we estimated a sam-
TNT, 2,4-DNT, and 2,6-DNT in a similar manner at 4
and -12C, using SARM-grade material. Effective
pling volume of 43.1 mL for the 20-minute sorp-
tion period, or an effective sampling volume of
sampling rates for these compounds as a function
2.16 mL/min at 23C. This volume is similar to the
of temperature are presented in Table 7.
12
To Contents




 Previous Page
Previous Page
