eq 1, we obtained a sampling volume of 46 mL for
a GC-NPD (SRI Instruments Model 8610).
a 20-minute exposure period. Since the amount
The mass of each compound was determined
sampled is linearly related to exposure period, the
against SARM-based standards prepared in ace-
tone.
effective sampling rate for TNT with the poly-
acrylate fiber is about 2.3 mL/minute at 23C.
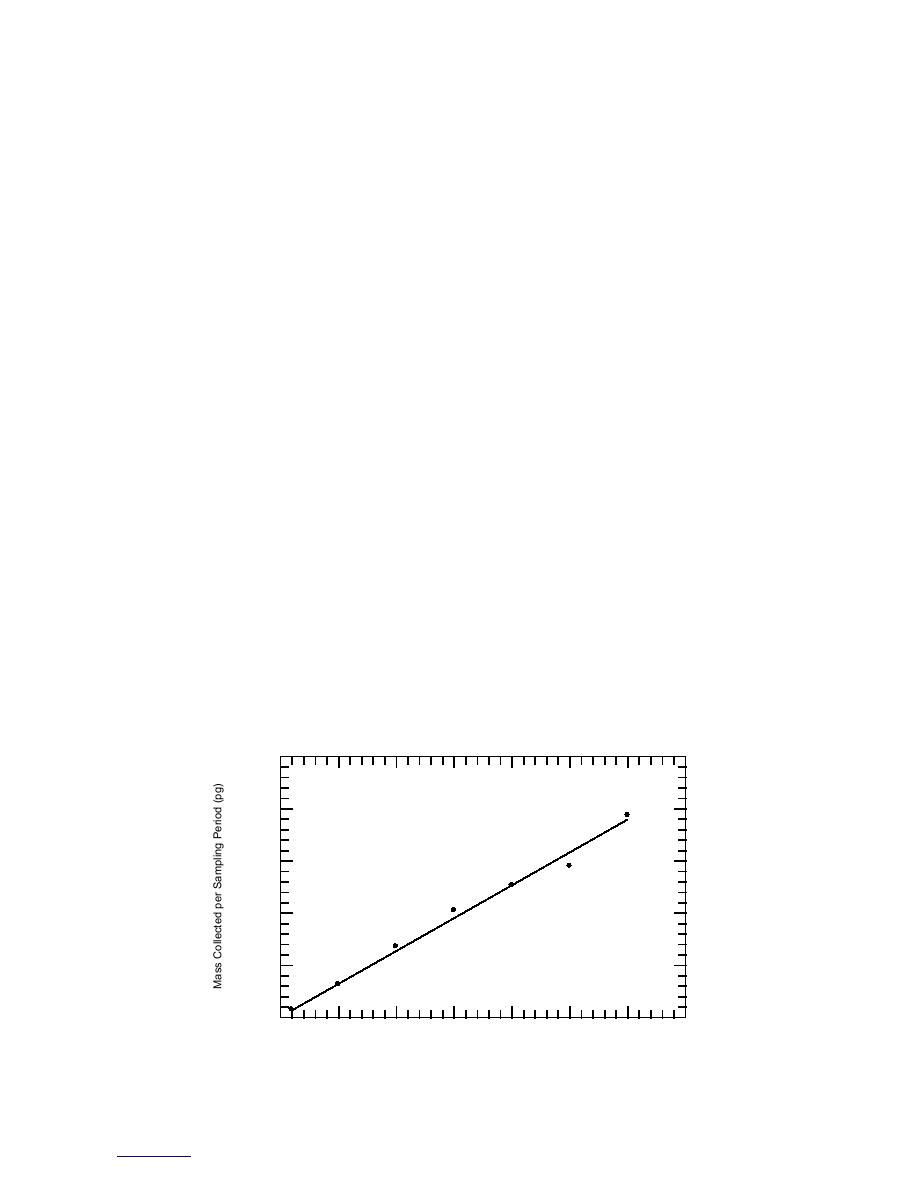
A plot of the mass of 2,4-DNT recovered from
the fiber as a function of sorption time is presented
Since the three major vapor signature chemicals
in Figure 9. Inspection of the data indicates that a
from military-grade TNT are 2,4-DNT and 2,6-
linear model with zero intercept adequately
DNT (Murrmann et al. 1971, Leggett et al. 1977),
describes them over the entire sorption period
and 1,3-DNB (discussed above), it is also impor-
tested. The slope of the linear model between
tant to be able to calculate the vapor concentra-
mass sorbed (ng) and sorption time is 6.30, with a
tions for these substances. In order to do so, we
correlation coefficient of 0.989. When we solved
need to know the effective sampling rate for these
this equation for the mass sorbed at 20 minutes,
compounds using the polyacrylate fiber. The
we obtained 126 ng. Using this value in eq 1, along
vapor pressures of 2,4-DNT, 2,6-DNT, and 1,3-
DNB are 1.44 104, 3.71 104 (Pella 1977), and
with the equilibrium vapor concentration calcu-
lated from the vapor pressure (1.424 109 g/mL),
6.22 10-4 torr (Howard and Maylan 1997, ex-
trapolated) at 22C, respectively, and the resulting
we calculated a sampling volume of about 88.5
vapor concentrations are 1.42 109, 3.67 109, and
mL for a 20-minute sampling time, or an effective
5.68 109 g/mL, respectively. These concentra-
sampling rate of 4.45 mL/min at 23C. This sam-
tions result in masses collected on the fiber that
pling volume is about double that obtained for
2,4,6-TNT at 23C.
are above the linear range of the ECD when
SPME exposure periods are more than a few min-
Similarly, plots of the masses of 2,6-DNT and
utes. The nitrogen-phosphorus detector (NPD) is
1,3-DNB recovered as a function of sorption time
selective for nitrogen-containing compounds, but
are presented in Figures 10 and 11. In both cases,
is not nearly as sensitive as the ECD. Thus, to
the mass recovered as a function of sampling time
calibrate the effective sampling rates for 2,4-DNT,
was adequately described by linear models with
2,6-DNT, and 1,3-DNB, 50-mg portions of SARM-
correlation coefficients of 0.992. When an effective
grade material for each compound were placed in
sampling volume for 2,6-DNT was computed, as
individual 40-mL amber vials and the headspace
described for 2,4-DNT, a sampling volume of 81.1
equilibrated for 7 days. The headspace above each
mL was obtained for a 20-minute sorption period,
which is 4.05 mL/min at 23C. This value com-
compound was sampled by exposing polyacry-
late SPME fibers to the respective headspace in
pares favorably with 4.45 mL/min for 2,4-DNT at
the vial for periods ranging between 1 and 30
the same temperature. Since these two species are
minutes and desorbing the fibers in the injector of
geometric isomers of one another, with the two
250,000
^
y = 6300x
200,000
2
r = 0.990
150,000
100,000
50,000
0
0
35
5
10
15
20
25
30
Sorption Time (min)
Figure 9. Mass of 2,4-DNT vapor sorbed on a polyacrylate SPME fiber at 23C.
11
To Contents




 Previous Page
Previous Page
