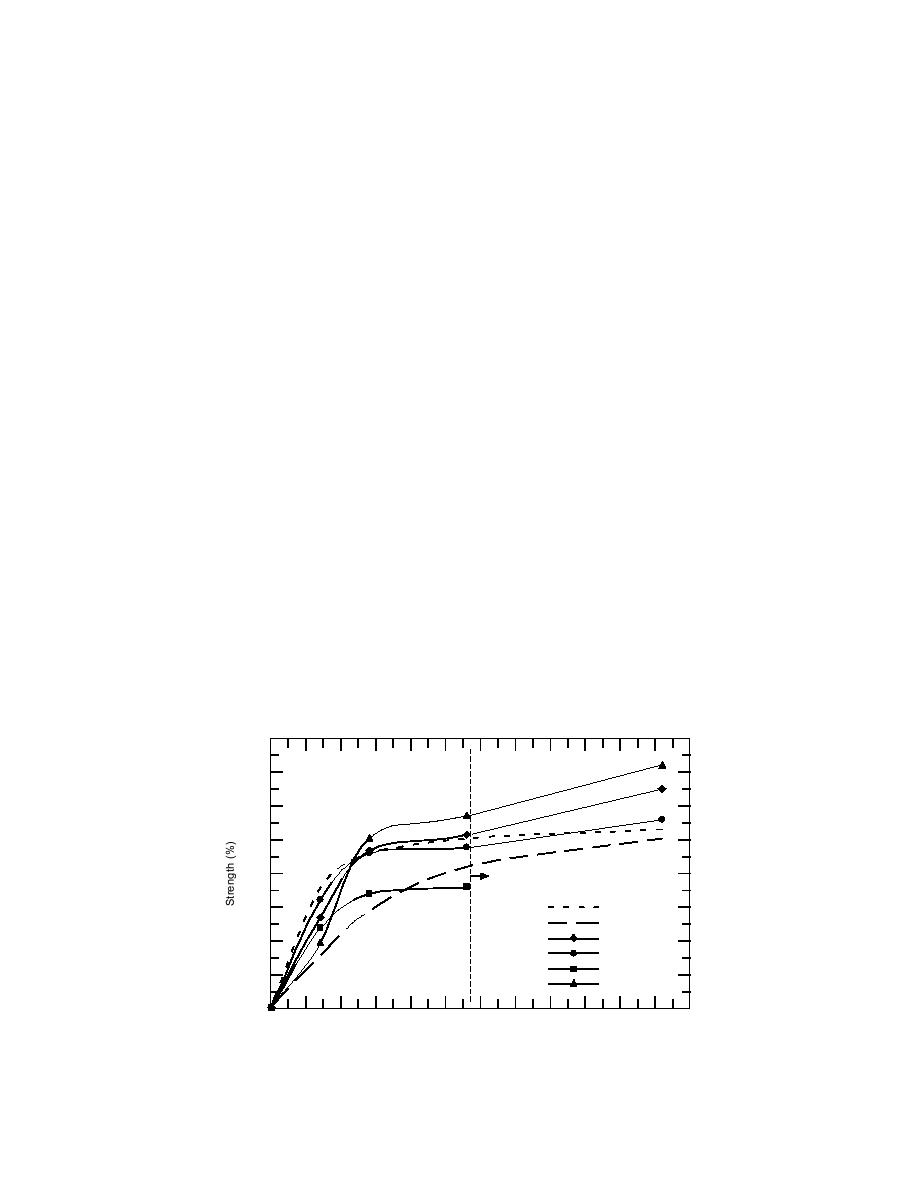
low temperatures. As seen, there is a scattering of
strengths developed by antifreeze mortars cured
the data points about the least-squares regression
at low temperature to those developed by normal
line drawn through them. This scattering is prob-
mortar cured according to current guidelines. In
ably caused by variations in attractive forces
practice, fresh mortar must be kept at or above
5C to ensure against freezing and assure a rea-
between particles and in solubility from chemical
to chemical. The least-squares line reveals that
sonably rapid strength gain. Therefore, the
each mole (mol) of solute reduces the freezing
strength-gain curve for control mortar cured at
point (FP) of the water by 1.76C. Further, it
5C is shown in Figure 2 as the benchmark for the
shows that admixture-free mortar should freeze
antifreeze mortars in this study. The curve for
at 1.28C. (The measured freezing point of the
20C mortar is shown for comparison. Any
control mortar was 1.3C.)
admixture that caused mortar, cured below 0C,
to gain strength at least as rapidly as control mor-
tar cured at 5C was deemed acceptable.
FP = 1.76 mol 1.28 .
(1)
Figure 2 shows four mortars that produced at
least benchmark strengths at 10C. Quite a few
Interestingly, the relationship between molali-
ty and freezing point remains quite good, even at
of the chemicals produced excellent results at
5C, while none passed the test at 20C. The
the highest dosage. Thus, eq 1 is a good first esti-
mate of expected freezing point whenever the
best single chemical was sodium nitrite. At a dose
chemical composition of the admixture is known.
of 9% by weight of cement, this chemical pro-
duced strengths at 10C that not only exceeded
However, it is always best to experimentally
determine the freezing point because, as noted,
the benchmark, they nearly equaled the strengths
of control mortar cured at 20C between 14 and 28
the colligative behavior of chemicals can vary
from that predicted by eq 1.
days. Thereafter, the sodium nitrite outper-
formed the 20C control mortar. This immediately
suggested that sodium nitrite, which is classified
The basic antifreeze admixture utilizes both
as a freezing point depressant in Table A2, could
accelerators and freezing point depressants.
be improved further by combining it with an
Other chemicals, such as plasticizers, retarders,
accelerator--something from Table A1.
and air entrainers, can be used, but this study did
Two accelerators provided that improvement.
not evaluate them. My objective was to find
Potassium carbonate and calcium nitrite each
affected sodium nitrite's performance at 10C
chemical combinations that promote "adequate"
strength gain while the internal temperature of
differently. (Calcium chloride was tested in the
the mortar was below 0C.
form of a commercial deicer along with sodium
One way to judge adequacy is to compare the
nitrate as a fertilizer. They did not perform as
160
140
120
100
80
20 Cure
Control 20C
60
Control 5C
SN
40
SN/PC
SN/SS
20
SN/CN
0
5
10
15
20
25
30
35
40
45
50
55
60
0
Time (days)
Figure 2. Best strengths at 10C.
5




 Previous Page
Previous Page
