the control mortar at 50C. Except for calcium
nitrite were the most active chemicals studied.
They produced 7-day strengths at 10C that
chloride and calcium bromide, the amount of
strength increase depended on the amount of
were comparable to control mortar cured between
5 and 10C. Since accelerators are only one com-
chemical added to the mortar. The calcium chlo-
ride and calcium bromide produced approxi-
ponent of an antifreeze admixture, these results
mately the same 7-day strengths despite differ-
are remarkable. Interestingly, too, the lowest
ences in doses.
measured freezing points of the mix waters were
6.2 and 5.7C for the calcium chloride and calci-
The best known cement hydration accelerator
is calcium chloride. As Table 2 shows, it provides
um nitrite, respectively. These are not insignifi-
more strength gain at a given dosage than the
cant from practical considerations as the control
mortar froze at 1.3C.
other accelerators. It is also the cheapest of all the
chemicals tested in this study. However, a major
drawback of calcium chloride is its tendency to
Freezing point depressants
corrode metal. As a result, calcium chloride is not
The second and final function of an antifreeze
recommended by current standards beyond 2%
admixture is to depress the freezing point of
by weight of cement in reinforced concrete and is
water. This function depends on a colligative
not recommended at all in pre-stressed concrete.
property called molality--the number of parti-
But, for expedient purposes where long-term
cles (moles) that dissolve into 1 kg of water. This
effects are less important or for non-reinforced
is a group of chemicals that are generally weak
structures where corrosion is not an issue, higher
accelerators or retarders, and includes both elec-
dosages are acceptable. Up to 4% was included in
trolytes and nonelectrolytes. Electrolytes are ma-
this study. For practical purposes, the amount of
terials that dissociate into more than 1 mole of
accelerator that can be added to concrete is limited
ions per formula weight, whereas nonelectrolytes
by how early the concrete stiffens before interfer-
remain in molecular form. Examples of electro-
ing with placing, consolidating, and finishing
lytes are sodium chloride and sodium acetate and
operations. Since ready-mix concrete is often pro-
of nonelectrolytes are alcohols and glycols. They
duced at temperatures around 20C during the
work on the principle that solute particles lower
winter, the accelerating potential of chemicals
the vapor pressure and, thus, the freezing point of
was judged at this temperature. Based on this
water.
study, the optimum dosage of accelerators
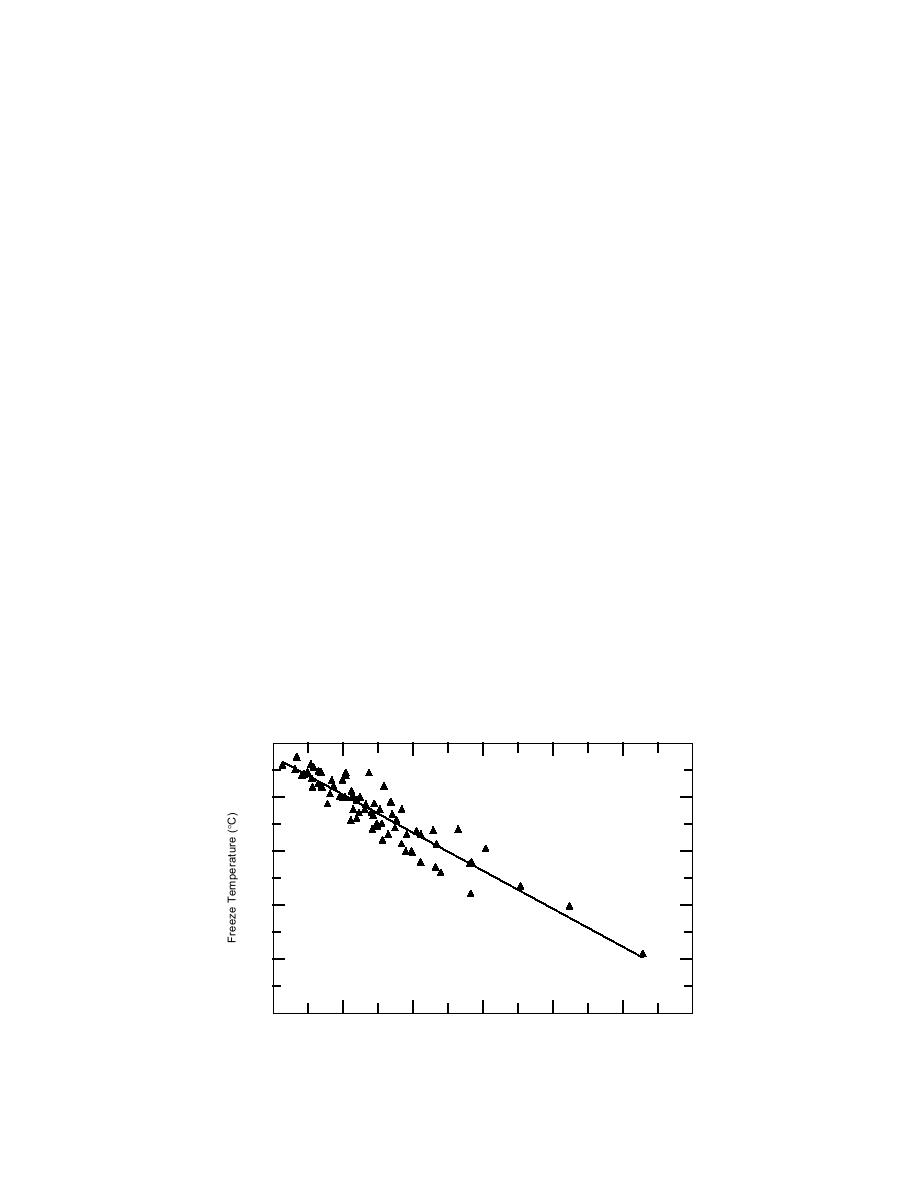
Figure 1, developed from Appendix A data,
appears to be between 4 and 8% for concrete
shows that, generally, the greater the number of
cured at 20C.
solute particles, the lower the freezing point. This
At lower temperatures, accelerator dosages
relation is independent of the type of chemical
can be higher. Calcium chloride and calcium
used, provided the chemical remains soluble at
0
-5
-10
-15
-20
-25
0
2
4
6
8
10
12
Concentration (molality)
Figure 1. Relationship between chemical dosage and freezing point.
4




 Previous Page
Previous Page
