200 mV lower than the pitting potential gives
an indication of possible cathodic inhibition.
+
The test results for two specimens for each
condition are shown in Table 9. The full test
3
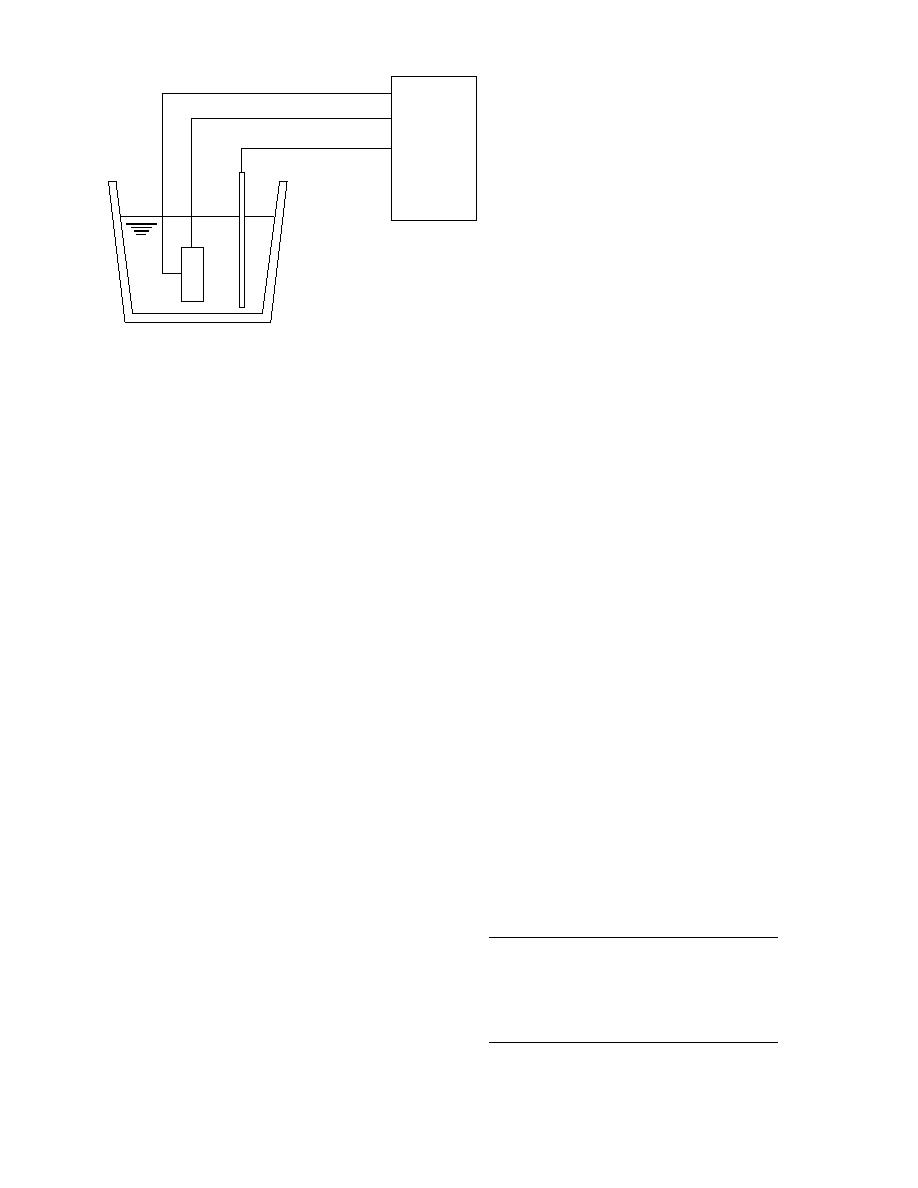
Potentiostat
charts are also included for reference for those
+
familiar with these electrochemical tests.
Specimens 1A and 1B were the control speci-
2
mens (no antifreeze admixture) tested in a
saturated calcium hydroxide solution also
containing sodium chloride at 5% by weight
of water, with no other concrete admixture
present. Notice that these specimens showed
1 = Steel Cylinder
1
2 = Counter Electrode
the most negative pitting potential (more
4
3 = Reference Electrode
prone to pit), as expected. Specimens 2A and
4 = Ca(OH) + Admixture (if applicable)
2B were like 1A and 1B, except that the anti-
Figure 2. Cyclic polarization test.
freeze admixture code-named DP was in-
dosages of set accelerators (i.e., > 6% s/s) formu-
cluded in the solution at 6% by weight of water.
lated with various calcium salts can prolong the
Notice that the pitting potential was much more
set of concrete compared to a control mix. In prac-
positive, which indicates less pitting tendency with
tice, set accelerators are rarely used above 4% s/s.
this antifreeze admixture.
The set times for the mixtures containing DP or
2) Lollipop test. This test measures the corro-
DPTC at 6% are similar to those of the mixture
sion that can occur in a steel rebar partially em-
containing PolarSet.
bedded in a concrete cylinder. The rebar protrudes
Task 2: Corrosion. Two types of tests, 1) cyclic
from the concrete cylinder, which is partially im-
polarization test, and 2) lollipop test, were con-
mersed along its longitudinal axis to half its height
ducted.
in a 3% sodium chloride solution. The initial resis-
tivity is measured using standard AC impedance
ing chemical admixtures (code names DP and
techniques.
DPTC) and a control were evaluated for their ten-
Six specimens for each admixture and a control
dency to pit by a cyclic polarization test. This test
solution were tested. The test results are shown in
subjects the surface of a metal to conditions pro-
Table 10. The data suggest that these admixtures
moting pitting in environments similar to those
reduce the corrosion rate compared to the control
found in concrete pore water. A 9-mm-diameter,
specimens. Therefore, from the standpoint of cor-
13-mm-long steel cylinder is immersed in a satu-
rosion potential, these admixtures do not lead to
rated calcium hydroxide solution with and with-
increased corrosion of embedded steel.
out sodium chloride (Fig. 2). The steel cylinder is
Task 3: Hydration products. Strength, durability,
polarized from 800 mV versus saturated calomel
and other concrete properties are affected by the
electrode (SCE) at a scan rate of 5 mV/s until the
composition and microstructure of the products
current reaches 255 A/cm
2, at which point the
formed during hydration. The composition, struc-
potential is reversed. The test ends at a potential
ture, and overall quality of a hardened cement
of 700 mV versus SCE.
paste are determined primarily by four factors: 1)
The test determines the pitting tendency of ad-
type and amount of cement, 2) the water/
mixtures. Three important data points are ob-
cementitious material ratio, 3) moisture availabil-
tained:
Eb = breakdown potential (the potential at
Table 9. Pitting potential.
which pitting starts).
Test
Solution [in addition to
Pitting potential
Ep = pitting potential (the potential below
no.
H2O and Ca(OH)2]
(mV)
which pitting cannot occur).
I = current density 200 mV below the pit-
1A
NaCl
550
ting potential.
1B
NaCl
525
2A
DP + NaCl
29
The more negative the values of Eb and Ep, the
2B
DP + NaCl
39
less effective is the admixture as a corrosion in-
3A
DPTC + NaCl
59
3B
DPTC + NaCl
80
hibitor. The magnitude of the current density at
8




 Previous Page
Previous Page
