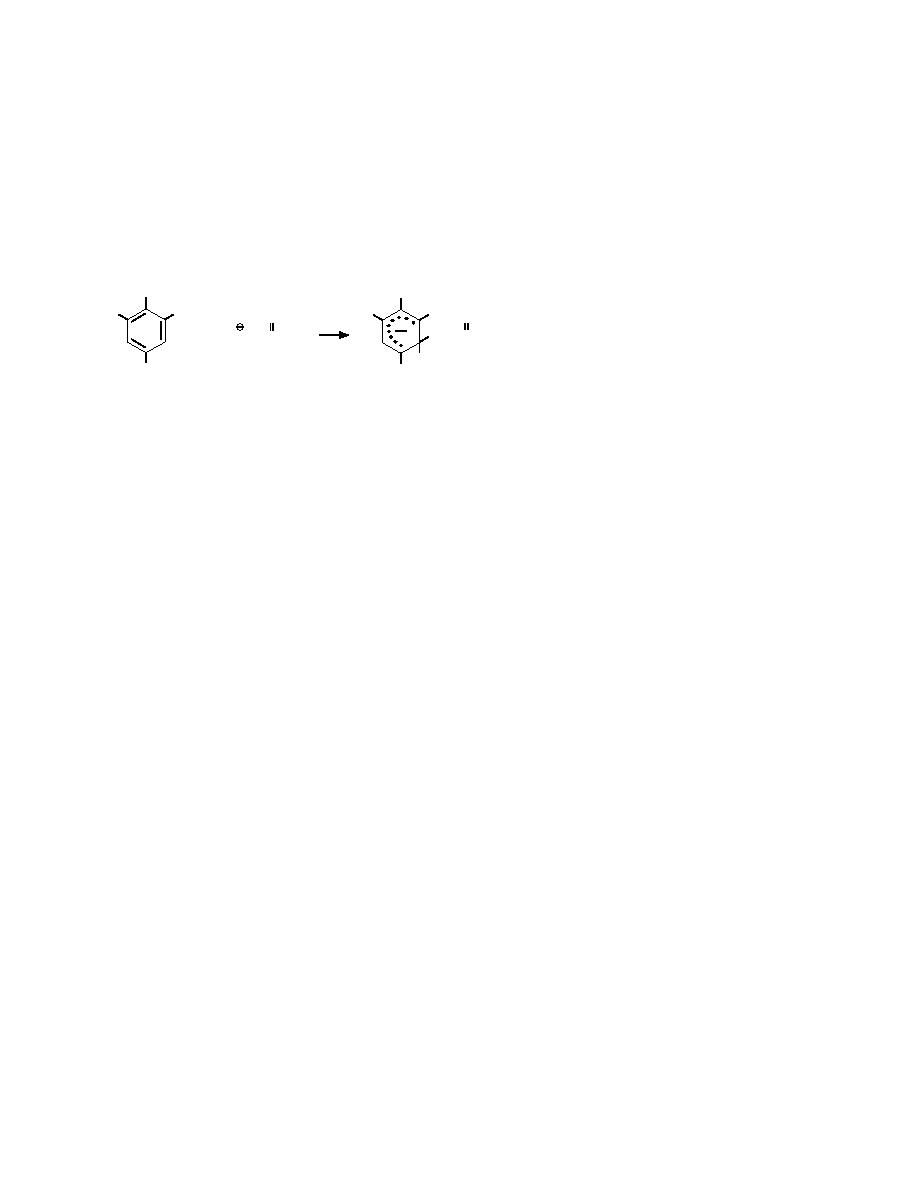
Colorimetric TNT method
are measured as parts-per-million (mg/kg) in soil
The colorimetric on-site method used for TNT
samples, in effective ranges between 0.5 and 5
analysis of acetone soil extracts is commercially
ppm for TNT and between 0.5 and 6 ppm for
available from EnSys. This is a commercialized
RDX. In the case where results higher than 5 ppm
version of the method developed by Jenkins (1990)
were obtained for TNT ("Hi" reading), dilution of
and utilizes the Janowsky reaction (eq 2). If TNT
the extracts were made by factors of two to four
is present in the acetone extracts, reaction with 1
in order to obtain a result within the effective
drop of the EnSys color reagent produces a pink
range of the test.
to red color indicative of the presence of the
Soils were extracted with acetone as described
Janowsky anion of TNT:
above and analyzed according to the instructions
provided with the D TECH TNT and
RDX explosives test kits. The same ac-
CH3
CH3
etone extracts used for the colorimet-
R
NO2
R
NO2
(2) ric on-site methods were used for EIA
O
O
+ CH2 -- C -- R
methods as well. A 1.0-mL aliquot of
CH2 -- C -- R
clear acetone extract was transferred
H
NO2
NO2
into a bottle of buffer solution (bottle 2
Janowsky Reaction (1891)
in the extraction pack). The prescribed
R = NO2 for 2,4,6-TNT
R = H for 2,4-DNT
volumes of the soil extracts were added
to the vials containing enzyme-labeled
A 25-mL aliquot of the acetone soil extract is
RDX or TNT and antibody-coated latex particles.
added to a 25-mL glass cuvette and the initial
Those mixtures were allowed to stand for 2 min-
absorbance measured with a Hach DR/2000 spec-
utes for the TNT test and 5 minutes for the RDX
trophotometer at 540 nm. A drop of the EnSys
test to allow the explosive molecules to interact
color reagent is then added to the cuvette and
with the antibody binding sites. Negative control
mixed by swirling. The solution is allowed to
references were processed with each analysis.
stand for one minute and then the absorbance is
Samples and references received identical treat-
again measured at 540 nm. Extracts were diluted
ments and both solutions were poured onto the
as appropriate, such that absorbances after reac-
respective sides (test or reference) of the porous
tion with the EnSys reagent were less than 1.0.
membrane cup assembly. The conjugate solutions
The concentration of TNT is estimated by sub-
were allowed to pass through the membranes,
tracting twice the initial absorbance from the final
and the membranes were washed and treated with
absorbance and dividing by the response factor
a color-developing solution. The reference sides
obtained from a TNT standard with a solution
of the cup were used to determine the end-point
concentration of about 4 mg/L. Doubling the
of the color development. The time for complete
initial absorbance prior to subtraction takes into
color development was less than 10 and 15 min-
account the increased absorbance caused by reac-
utes for TNT and RDX, respectively. All of these
tion of humic organics in the extract with base, as
manipulations and readings were done at room
discussed elsewhere (Jenkins and Walsh 1992).
temperature. RDX EIA tests were performed only
on the first set of samples, since it was clear that
D TECH immunoassay
HMX was the main contaminant, and cross-
for RDX and TNT
reactivity with RDX EIA test was not sufficient
The D TECH enzyme immunoassay (EIA)
to serve as a HMX evaluation tool. All samples
method used for both RDX and TNT is commer-
were tested with the TNT EIA method.
cially available from EM Science (Teany and
Results from the test kits were determined with
Hudak 1994, Teany et al. 1995). The components
the DTECHTOR environmental field test meter
of this EIA include RDX- and TNT- specific anti-
(EM Science). This device is a hand-held reflecto-
bodies covalently linked to small latex particles,
meter powered with a 9-V plug-in battery. It mea-
which are collected on the membrane of the cup
sures the amount of light reflected from the
assembly. A color-developing solution added to
surfaces of the color-developed test and reference
the surface of the cup assembly reveals a color
sides of the cup assembly. Readings are in per-
inversely proportional to the concentration of RDX
centages, which can then be translated into TNT
or TNT equivalent in the sample. RDX and TNT
or RDX equivalent concentration ranges.
7




 Previous Page
Previous Page
