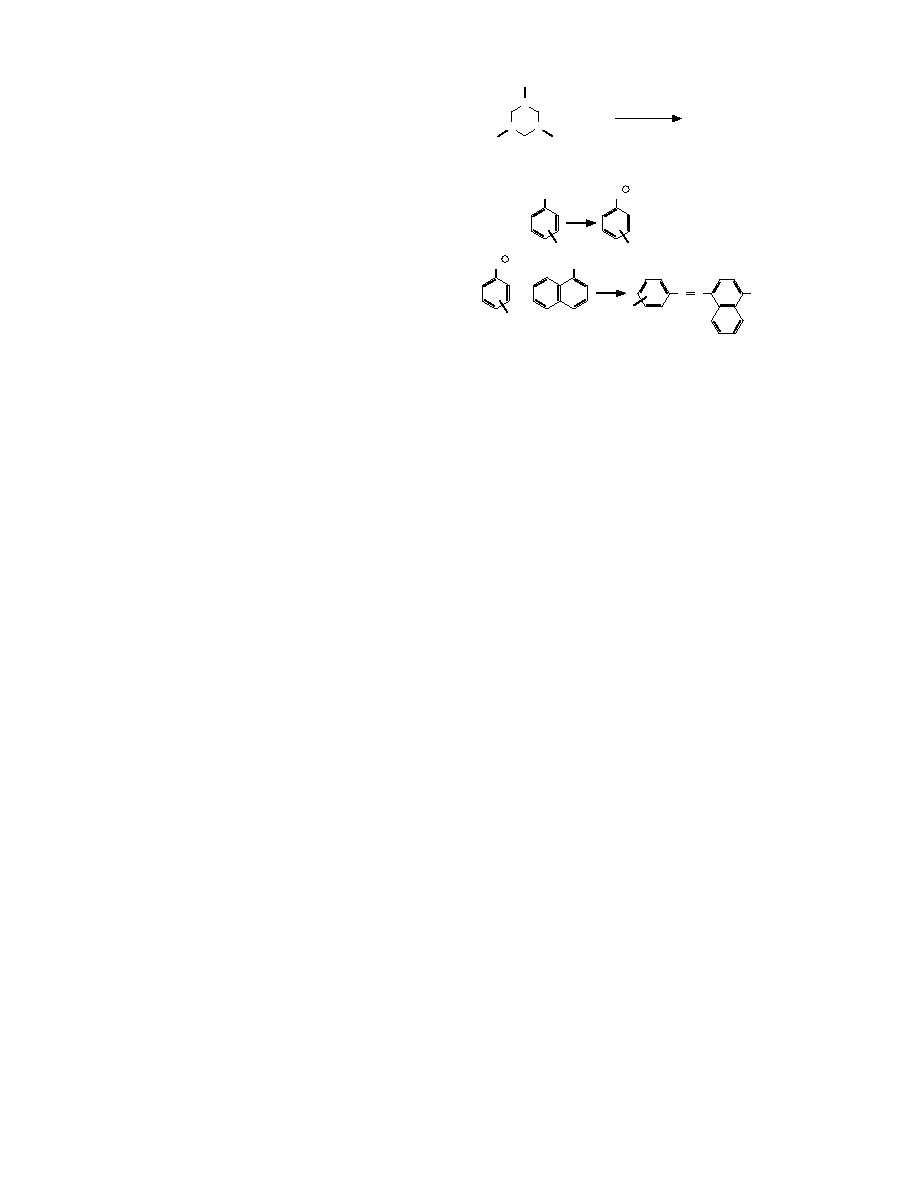
were collected for on-site analysis and for sub-
NO2
sequent laboratory analysis using Method 8330.
N
Acetic Acid
+
In all cases separate subsamples were used for
Zn
3 HNO2
N
N
moisture content determination, and analysis
O2N
NO2
results were corrected to a dry weight basis.
RDX
Franchimont Reaction (1897)
Weighed portions from each of the four area-
+
integrated subgrid samples from within a grid
NH2
N2
were combined and homogenized to prepare a
(1)
+
HNO2
composite sample to represent each grid. Dupli-
cate 20-g portions were collected for on-site analy-
R
R
+
sis of each composite grid sample, and also for
N2
NR'
2
area integrated subgrid samples for subgrids D2
+
NR'
N
N
and D5. We used the results from these dupli-
2
R
cates to compute analytical uncertainty, i.e., the
R
uncertainty associated with subsampling, extrac-
Azo Dye
tion and analysis.
Griess Reaction (1864)
On-site sample
Extracts were analyzed as described elsewhere
extraction and filtration
Each 20-g soil subsample was extracted with
(Walsh and Jenkins 1991) except that the extracts
100 mL of acetone. Based on a short extraction
were not passed through an anion exchanger
kinetic study with the initial samples collected, a
prior to further processing. The use of the anion
30-minute extraction on a vortex mixer was
exchanger is required to remove interferences
needed, rather than the 3-minute period specified
from nitrate and nitrite ions, often present in
by the manufacturers of the on-site tests. The ex-
areas where soils have been given chemical
tracts were allowed to settle for at least 30 min-
fertilization. Since the history of this site was
utes and a 50-mL aliquot was removed from the
known and fertilization had not been used, the
extraction bottle with a disposable Plastipak
anion exchange step was eliminated.
syringe and filtered through a Millex SR filter
A 5.0-mL aliquot of each acetone extract was
membrane. All samples collected by the joint
mixed with 0.5 mL of glacial acetic acid and
Canadian-United States team were analyzed
poured into the barrel of a 10-mL syringe loaded
using the TNT and RDX methods by EnSys Cor-
with 0.3 g of zinc dust and equipped with a Millex
poration (now Strategic Diagnostics, Inc.). Some
SR-filter unit. The plunger was fitted to the barrel
of the soil extracts were also analyzed using the
of the syringe and after 15 seconds of contact with
the zinc, the solution was filtered into a vial
D TECH enzyme immunoassay TNT and RDX
containing 20 mL of deionized water and the con-
methods (EM Science). The acetone extracts were
tents of a Hach NitriVer 3 powder pillow. The
subsequently returned to CRREL and analyzed
vial was shaken to mix and allowed to stand for a
using RP-HPLC separations similar to those
minimum of 15 minutes.
described in SW846 Method 8330 (EPA 1995).
The development of a pink color is indicative
of the presence of either a nitramine such as RDX
On-site analytical methods
or HMX, or an organonitrate ester such as nitro-
glycerine (NG), nitrocellulose (NC) or penta-
Colorimetric HMX method
erythritol tetranitrate (PETN). At the tank firing
The colorimetric method used to estimate HMX
range at CFB-Valcartier, the only munition fired
at CFB-Valcartier was originally developed for
RDX (Walsh and Jenkins 1991) and is now com-
is an antitank device containing HMX and TNT,
mercially available through EnSys as their RDX
and so the development of a pink color here
field method. When RDX is either not present, or
indicates the presence of HMX. Concentrations
present at a much lower concentration than HMX,
of HMX were estimated by measuring the ab-
this method can be used to estimate HMX con-
sorbance at 507 nm using a Hach DR/2000, a
centrations (Jenkins et al. 1995). The method is
battery-operated spectrophotometer. If absorbance
based on the Franchimont and Griess reactions as
values were above 1.0, extracts were diluted with
shown in eq 1:
acetone (3% water added) and re-analyzed.
6




 Previous Page
Previous Page
