1200
Glycolate
Acetate
Formate
Oxalate
Glyoxylic
Pyruvic Acid
800
Unknown 1
Pyruvaldehyde
4 - ADNT
C Balance
400
0
4000
8000
12000
Time (s)
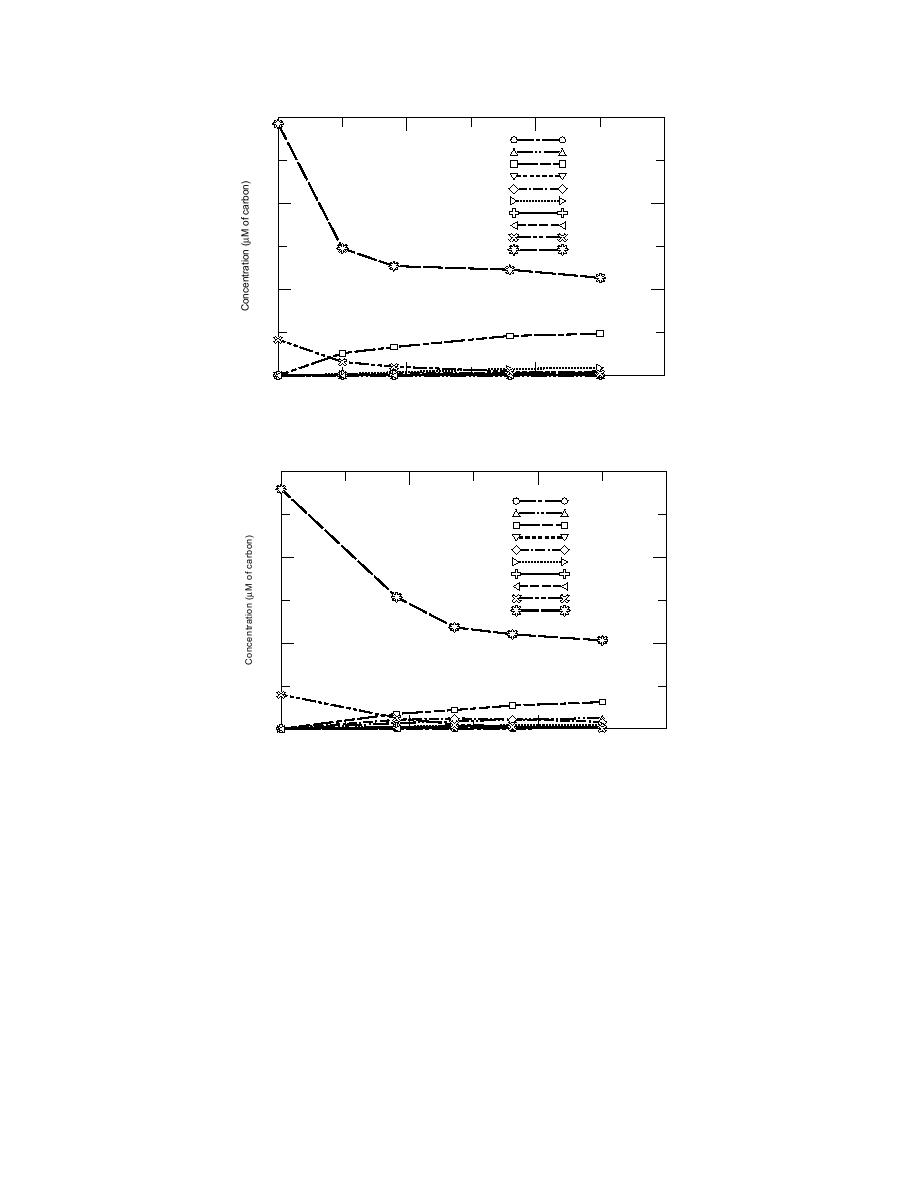
Figure 10. Carbon balance and product distribution from the ozonation of
2-ADNT.
1200
Glycolate
Acetate
Formate
Oxalate
Glyoxylic Acid
800
Pyruvic Acid
Glyoxal
Pyruvaldehyde
2-ADNT
C Balance
400
12000
0
4000
8000
Time (s)
Figure 11. Carbon balance and product distribution from the ozonation
of 4-ADNT.
sumed appear in Figure 12. Initially, nitrite concentrations are higher than nitrate; however, nitrite
will compete with the ADNT for ozone, resulting in its conversion to nitrate. A typical chromato-
gram for nitrite and nitrate appears in Figure 13.
DISCUSSION
ADNTs react rapidly with ozone and hydroxyl radical oxidants in aqueous systems. The perox-
one treatment of ADNTs will be controlled by ozone oxidation based on the measured rate con-
stants and relative oxidant concentrations in peroxone systems. Because of the magnitude of the
rate constants (105 M1 s1 for ozone and 109 M1 s1 for hydroxyl radical), competitive oxidation
kinetics offers a convenient method to study the reaction kinetics.
The rate constants for the oxidation of 2-ADNT and 4-ADNT by ozone were calculated to be 5.3
105 M1 s1 and 1.9 105 M1 s1, respectively, when nitrite was used as a reference chemical. The
rate constants for the oxidation of 2-ADNT and 4-ADNT by hydroxyl radical were calculated to be
9




 Previous Page
Previous Page
