0.08
436.9 nm
Glyoxal
4-ADNT Product
Pyruvic Aldehyde
0.06
209.7nm
AU
0.04
422.4 nm
432.0 nm
369.4 nm
219.1 nm
0.02
209.7 nm
0
200
300
400
500
600
Wavelength (nm)
Figure 6. UV spectral data for 4-ADNT product compared to glyoxal
and pyruvic aldehyde.
5
3
4 - Glyoxylic
4 - Pyruvic
4
4 - Derivative
4 - Pyruvalde
2
3
Glyoxylic
Pyruvic
Glyoxal
Pyruvalde
2
1
1
0
40
80
120
160
200
40
80
120
160
200
0
Time (min)
Time (min)
a. 2-ADNT.
b. 4-ADNT.
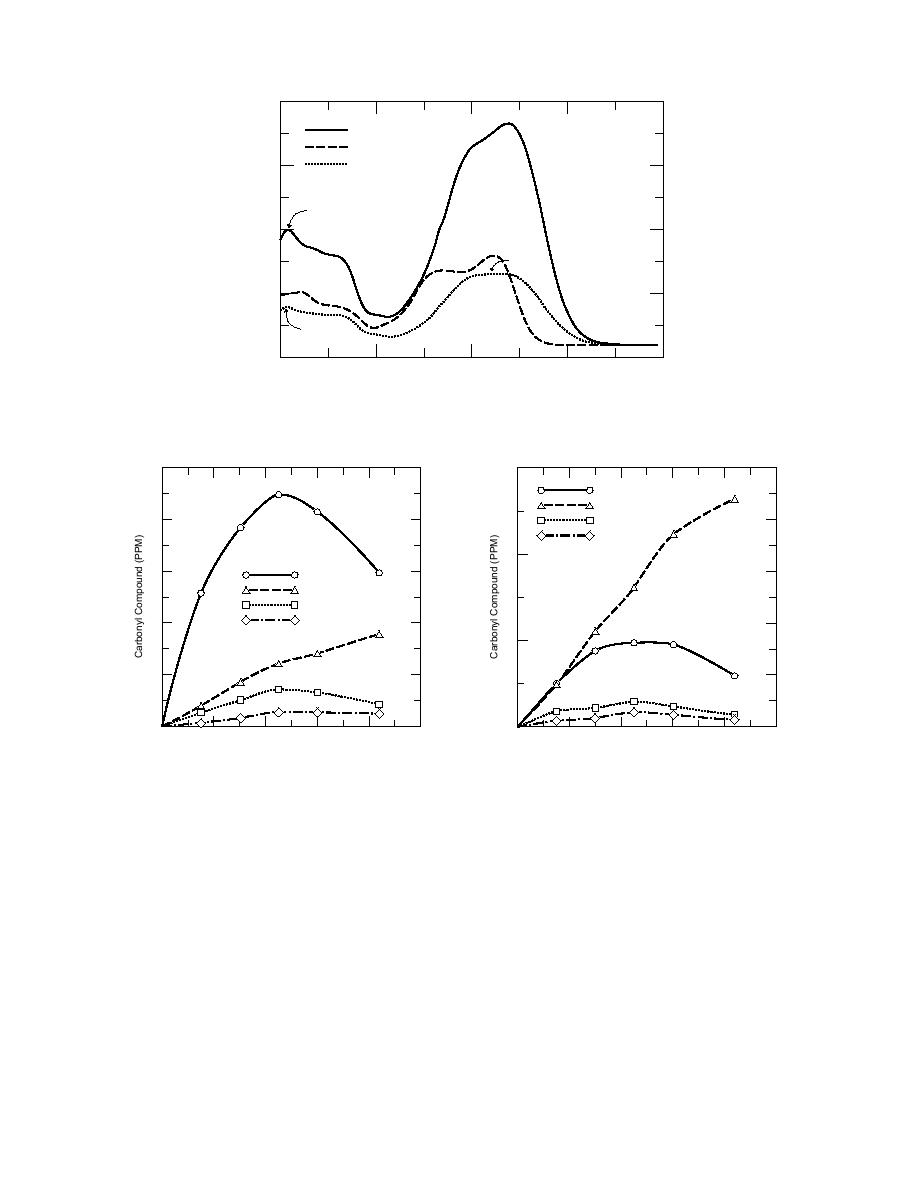
Figure 7. Quantitative determination of 2,4-dinitrophenylhydrazone derivatives of products from 2-ADNT
and 4-ADNT.
confirmed the generation of pyruvic acid as a stable end-product. Based on chromatographic re-
tention times, glycolic acid (HOCH2COOH) and acetic acid were also identified. Other acids ap-
peared in the profile, but their retention times could not be matched with authentic standards.
Oxalic acid eluted near the dead volume and was masked by the large amounts of nitrate ion
generated during ozonation. A comparison of acids generated from 2-ADNT and 4-ADNT is
shown in Figure 8 and indicates the direct difference in product compositions for identical ozona-
tion times.
Using anion exchange with conductivity detection, nitrate ion could be resolved from oxalic
acid and other carboxylic acids. Formic, malonic, oxalic, and acetic acids were observed. The tenta-
tive identifications of acids observed by this technique are shown in Figure 9 for 2-ADNT and 4-
ADNT. The product profile was found not to agree with that observed on the Supelcogel column
(with UV detection), especially with respect to formic and pyruvic acids. These results indicate
that other methods of confirmatory analysis need to be addressed for the carboxylic acid fraction.
7




 Previous Page
Previous Page
