Table 1. Loss of 2-ADNT and 4-ADNT (M) with
below), which indicates that hydroxyl radical
ozone in the presence of resorcinol and nitrite
plays an insignificant role in the oxidation of
reference chemicals.
ADNTs by ozone.
Rate
Rate
Resorcinol constant 2-ADNT Nitrite
constant
Direct hydroxyl radical rate constant (kHO)
(M)
(M)
(M)
(M1 s1 )
(M1 s1)
Most organics are oxidized by hydroxyl
2-ADNT
radical with rate constants in the range of 109
10.5
15.5
200
200
to 1010 M1 s1 (Buxton and Greenstoch 1988).
8.0
11.8
148
164
5.8
8.2
120
142
We selected PNAP as a reference compound
5.3 105
4.0
5.0
93.4
122
with an average hydroxyl radical rate
1.4 105
2.7
3.0
constant of 3.1 109 M1 s1 (Buxton and
4-ADNT
Greenstoch 1988). Average data (two deter-
11.0
14.5
300
300
minations) for the loss of 2-ADNT and
9.4
11.8
267
265
8.3
9.9
233
183
4-ADNT with respect to PNAP are shown in
1.5 105
1.9 105
6.1
6.0
192
139
Table 2.
0.2
0
0.2
0.4
0.6
0.8
2ADNT
1.0
4ADNT
1.2
0
0.2
0.4
0.6
0.8
ln[NO2]/[NO2]0
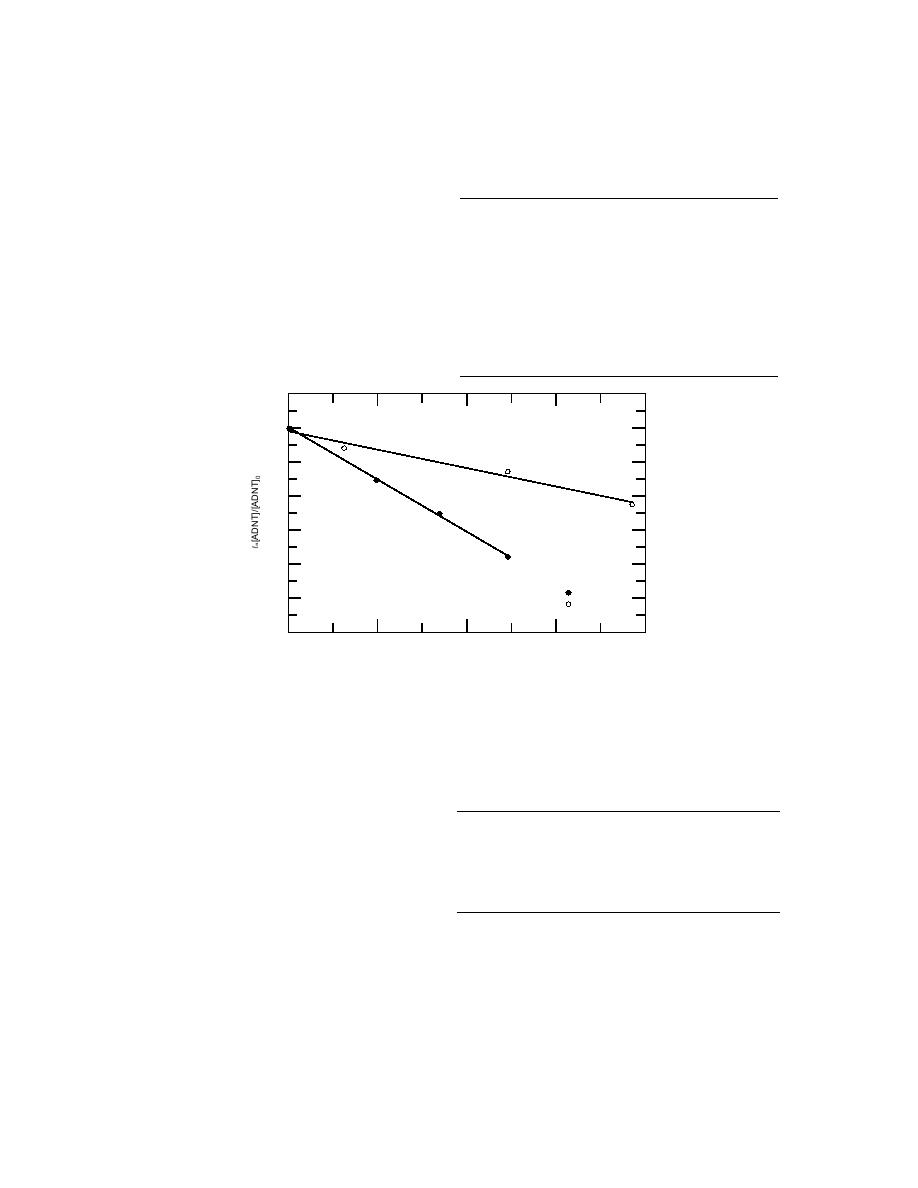
Figure 1. Measured ozone rate constants for 2-ADNT and 4-
ADNT against the reference chemical, nitrite.
Plots of the loss of ADNTs and PNAP
Table 2. Loss of ADNTs in the presence of hydroxyl
radical.
appear in Figure 2. From eq 4, hydroxyl radi-
cal rate constants were calculated to be 1.6
Rate
Rate
109 M1 s1 and 1.9 109 M1 s1 for 2-ADNT
2-ADNT
PNAP
constant 4-ADNT
PNAP
constant
(M)
(M)
(M)
(M)
(M1 s1 )
(M1 s1)
and 4-ADNT, respectively.
20.0
37.2
20.0
37.2
18.7
31.5
18.8
33.1
Oxidation in peroxone oxidizing system
16.7
24.9
17.3
28.9
The reactions of the ADNTs with peroxone
14.9
20.0
15.6
22.2
(O3/H2O2), ozone, and ozone with t-butyl
13.7
17.4
13.6
19.3
1.9 109
1.6 109
12.6
14.7
12.4
16.6
alcohol are depicted graphically in Figure 3.
These linear plots of ADNT consumed versus
ozone utilized indicate that hydroxyl radical, hydrogen peroxide, or the hydroxyl radical scaven-
ger (t-butyl alcohol) have little effect in the peroxone oxidation. These data suggest that the direct
oxidation by ozone controls the oxidative process. In studying the stoichiometry of the transfor-
mation in the above three systems, we found that each mole of 2-ADNT consumes 2.5 moles of
ozone. For 4-ADNT, the ratio was found to be similar: 2.7 moles ozone for each mole of 4-ADNT. If
we consider that one mole of ADNT will react with one mole of ozone, then ≈ 60% [(2.51.0)/2.5
100] of the ozone is used to oxidize secondary products generated in the transformation, and these
products are more reactive than the ADNT.
4




 Previous Page
Previous Page
