intermediate products make the study of the mechanistic degradation pathway a challenging en-
deavor. With the information gained in this study and the current understanding of the chemistry
of ozone in aqueous solution, one approach to evaluate pathways is to examine stable end-prod-
ucts and assess how these products may arise.
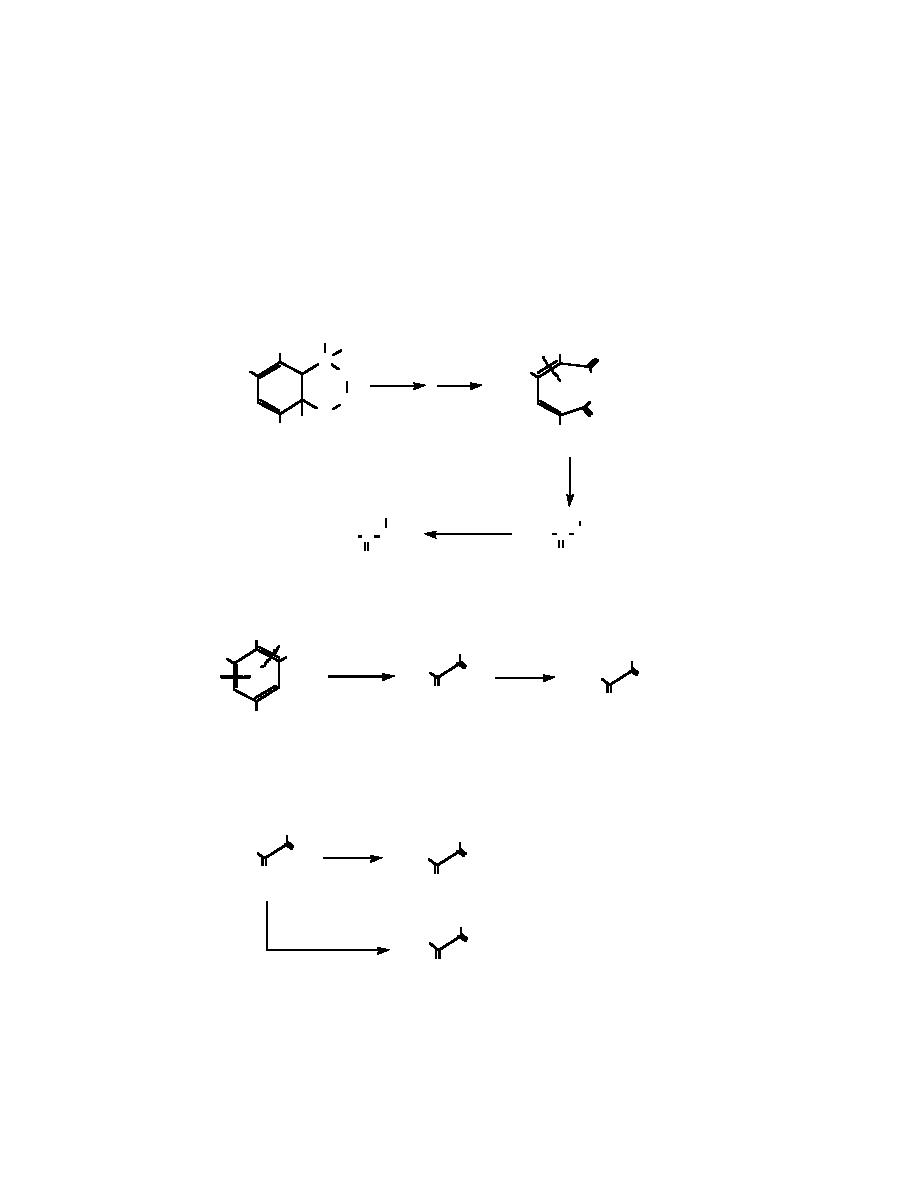
Pyruvic acid is generated as a major stable end-product from both 2ADNT and 4-ADNT. The
most direct route of formation involves cleavage around the aromatic methyl group as shown
below. We propose an ozone-amine adduct as an initial binding step, which ruptures the ring at
the 2-3 carbon bond. In a series of steps, nitrite is produced, which is observed early in the oxida-
tions. This pathway would generate pyruvic aldehyde (a found intermediate), which undergoes
further oxidation with ozone to produce pyruvic acid.
H
CH
H
CH 3
3
O
N
O2 N
O N
+
-
O
2
H
+
NO
2
H
O
O
O
H
NO2
NO2
OH
O
H
3
O
+
CH C C=O
CH3 C C=O
3
2
O
O
Cleavage on the right side of the carbon atom bearing the methyl group could also lead to pyruvic
acid.
CH3
CH3
O2 N
NH2
CH3
O3
H2O
O2 N
O
HO
+ HNO
O
3
O
O
NO 2
(I)
(I)
At this time, the behavior of the nitro intermediate (I) is not clear. While hydrolysis is expected,
reduction, leading to pyruvic aldehyde and nitrate, cannot be ignored as an alternate route of
transformation.
CH3
CH3
H2O
O 2N
O
+
HNO2
(Hydrolysis)
HO
O
O
O
CH 3
H2O
H
+ HNO3
(Oxidation/Reduction)
O
O
In the current investigation, a potential transformation product studied was oxalacetic acid.
This acid formed a 2,4-dinitrophenylhydrazone derivative that could not be found in ozonized
solutions. However, on standing, this derivative was converted to pyruvic acid. Therefore, it can-
not be ruled out that oxalacetic acid is a precursor to pyruvic acid.
11




 Previous Page
Previous Page
