120
phase extraction (SPE) using Por-
apak RDX, and membrane SPE us-
ing Empore SDB membranes, ac-
pH 2.0
cording to the methods described in
detail in Jenkins et al. (1994). The
pH 3.5, 2.5% ACN
resulting extracts were analyzed us-
7.5% ACN
80
ing RP-HPLC as specified in Method
8330, and percent recoveries were
calculated vs. initial spiked concen-
trations (Table 3). As you will note,
the results for the membrane SPE
method for the solution preserved
40
with 7.5% ACN indicated that re-
Unpreserved
coveries of the analytes ranged from
Control
only 3% for HMX and RDX to 45%
for 2,4-DNT. Since these recoveries
are unacceptably low, no additional
experiments were conducted using
0
20
40
Storage Time (days)
this preservative.
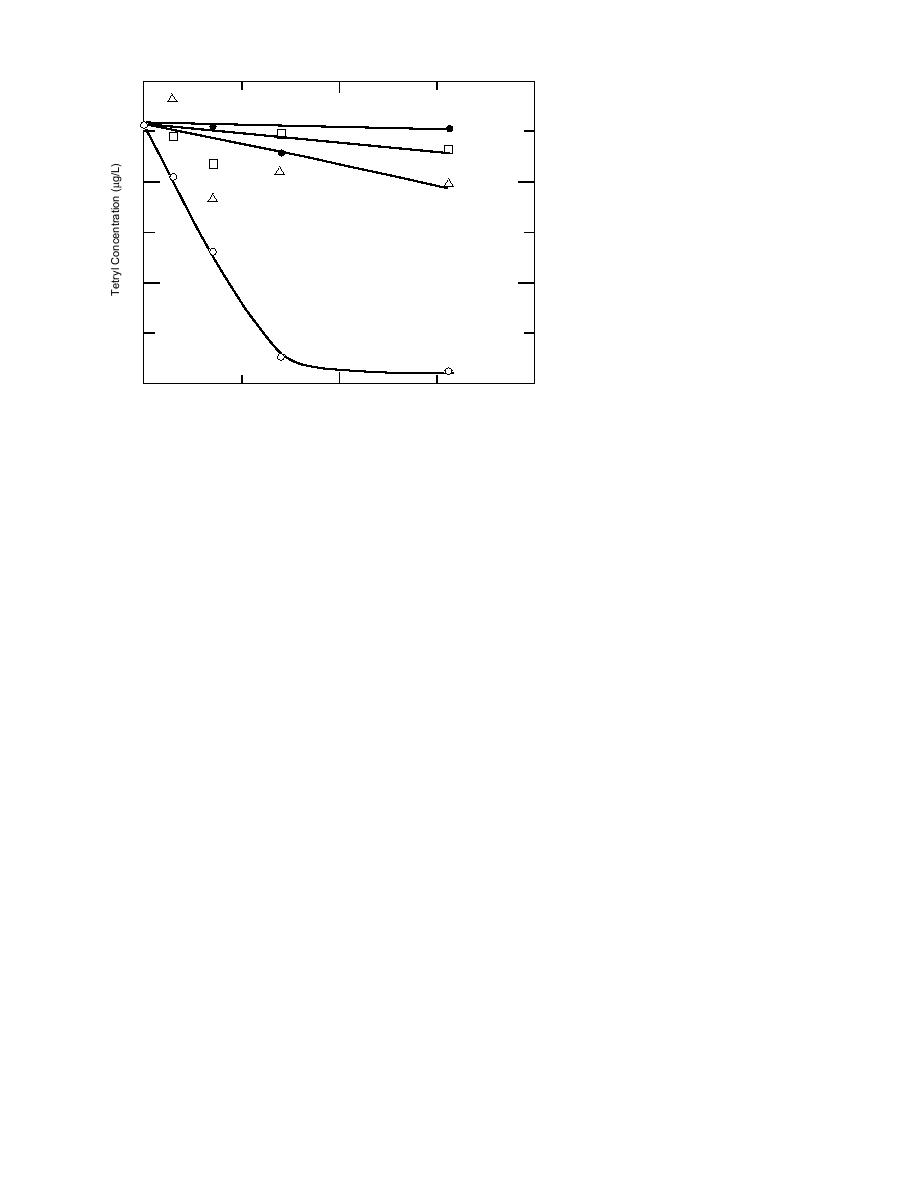
Figure 7. Stability of tetryl in samples preserved at pH 2, pH 3.5, with
Results for the SOE procedure,
2.5% acetonitrile, and with 7.5% acetonitrile.
when samples were acidified to pH
2, indicated that low recovery was
obtained for the two amino-containing analytes,
tralization. Low recovery could be due to some
3,5-DNA (35%) and 2ADNT (54%). These results
protenation of the amines at pH 2, although pKa
are consistent with some protenation of these com-
values for 2ADNT and 4ADNT have been reported
pounds to form the corresponding ammonium
as 0.59 and 1.23, respectively (Glover et al. 1977),
cations, which would not be expected to partition
indicating that most of both compounds should
favorably into the salted-out acetonitrile. A fur-
exist in the nonionized amino form at pH 2. Over-
ther test of the SOE procedure was conducted
all, the three stabilization techniques appear to be
quite adequate in preserving these four analytes.
when the initially pH-2-preserved solution was
neutralized with aqueous KOH to pH 6.6 before
extraction. The recovery of 3,5-DNA and 2ADNT
Evaluation of
improves to 100 and 97%, respectively, after neu-
selected preservatives on
tralization (Table 3). No problems were encoun-
EPA Method 8330 determination
tered with the pH-2-preserved solution with ei-
Effects on preconcentration methods
ther the cartridge or membrane SPE methods,
The next phase of this study was to evaluate
where recovery of the amino-containing com-
the effects these three potential stabilization tech-
pounds appears to be unaffected by the low pH.
niques have on the most commonly used analyti-
Recovery of HMX using the SDB membrane
cal method for nitroaromatics and nitramines,
method, however, is low for both preserved solu-
SW846 Method 8330. The following experiment
tions and the unpreserved solution, as was ob-
was conducted to determine the effects of these
served in our earlier study (Jenkins et al. 1992,
preservatives on the extraction/preconcentration
1994). An apparent high recovery of RDX was
procedures used for low-level determination.
found for the cartridge SPE method for all three
A solution was prepared containing HMX,
solutions. This again had been observed previ-
RDX, TNB, DNB, 3,5-DNA, TNT, 2ADNT, and 2,4-
ously; part of the problem appears to be due to a
DNT in reagent-grade water at concentrations
narrowing of the peak width for RDX for cartridge
ranging from 10.7 to 12.2 g/L. The solution was
SPE extracts compared with the unextracted stan-
divided into four portions; three were preserved
dards used for establishing response factors. All
as described above, and the fourth was left
three extraction methods appear to give good re-
unpreserved to serve as a control for comparison
covery for the portion of solution preserved with
of analytical results. Aliquots of each portion were
2.5% ACN at pH 3.5.
immediately preconcentrated using salting-out
These results indicate that acidification using
solvent extraction with acetonitrile, cartridge solid-
sodium bisulfate to pH 2 or addition of ACN to
9




 Previous Page
Previous Page
