manner similar to that described by Grant et al.
acid, and the pH of the remaining subsample at
(1993). Since TNB was the least stable analyte in
each ACN concentration was left unmodified.
the earlier study, it was selected as the test analyte
These 14 samples were stored and analyzed as
and was fortified at 50 g/L. Results indicated
described above. A final subsample of the forti-
that after three days' storage at room tempera-
fied Connecticut River water was acidified to pH
ture, the concentration of TNB was reduced by
2.0 with sodium bisulfate and held under refrig-
80% and a buildup of 3,5-DNA had occurred. Thus,
eration for 30 days. This sample was analyzed in
as observed previously, fortified Connecticut River
the same manner as described above after 0, 4, 8,
water should be an excellent test matrix for the
16, and 30 days of storage.
assessment of various stabilization techniques.
Analytical results for the control sample agreed
with those presented in Grant et al. (1993) for
nitroaromatic and nitramine fortified Connecticut
Preliminary evaluation of sodium
River water. The rate of loss of TNB was found to
bisulfate and percentages of acetonitrile
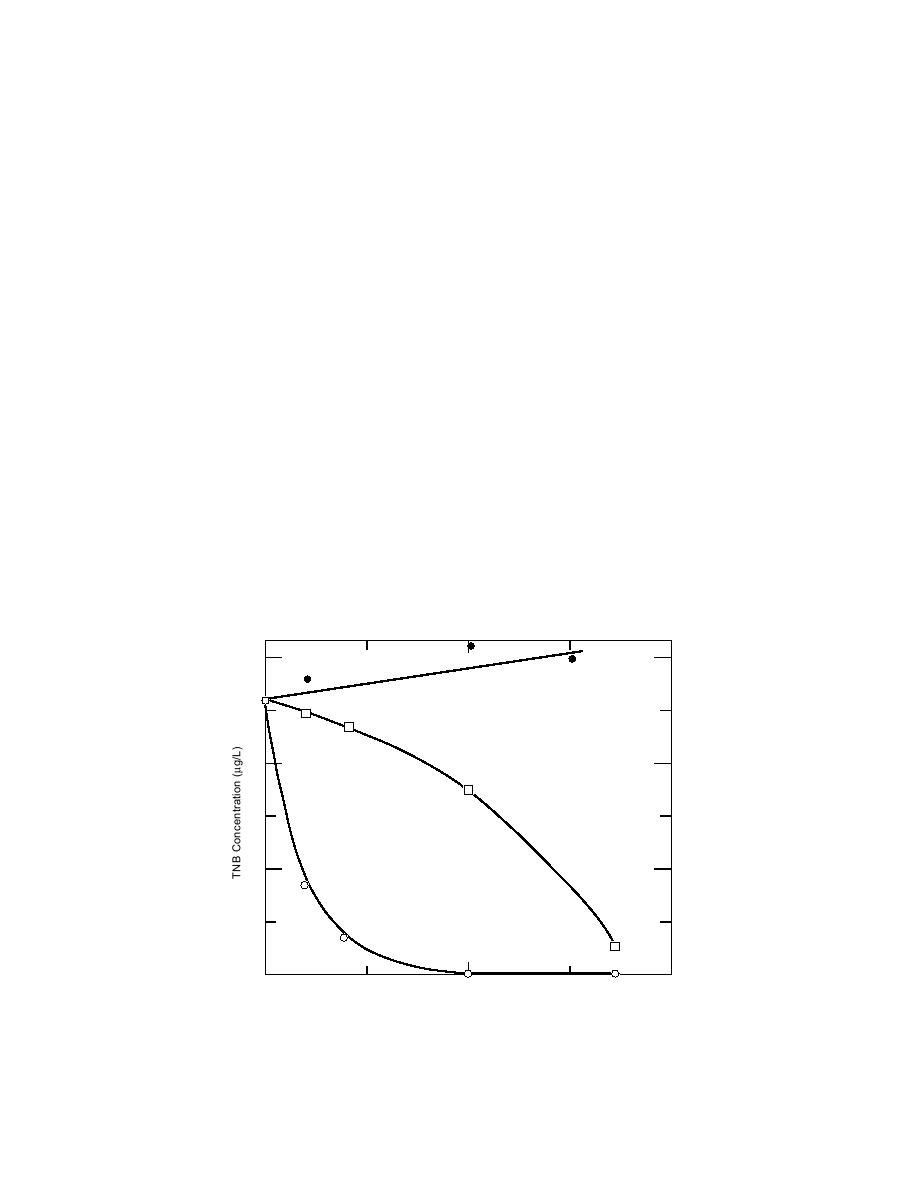
be very rapid (Fig. 1), the rate of loss of TNT was
necessary to stabilize samples
Connecticut River water was fortified with
somewhat slower, and there was no evidence for
RDX, TNB, and TNT at 42, 26, and 41 g/L, re-
loss of RDX. After only four days, the concentra-
spectively. One subsample of the fortified water
tion of TNB in the unacidified control sample was
reduced from 26.0 g/L to 8.3 g/L. The concen-
was stored under refrigeration without addition
of any chemical preservative, and this sample
tration of 3,5-DNA, the associated, relatively stable
served as the control sample for judging the effec-
transformation product, increased as the TNB was
lost, with a maximum concentration of 6.1 g/L
tiveness of the various chemical stabilization pro-
cedures examined. The water was analyzed at day
at 20 days (Fig. 2). The rate of loss of TNB was
0, 4, 8, 20, and 34. A second subsample was acidi-
reduced by acidification to pH 3.5 with acetic acid
fied to pH 3.5 with acetic acid and stored over the
(Fig. 1), and 3,5-DNA was not detected in this
same period under refrigeration. Acetonitrile
sample until day 20. By day 34, however, the TNB
concentration had been reduced to 2.7 g/L, which
(ACN) was added to 14 subsamples in the appro-
priate amounts to achieve acetonitrile concentra-
is about a 90% reduction in concentration relative
tions in duplicate at 0.1, 0.5, 1.0, 2.5, 5.0, 7.5, and
to day 0, and the 3,5-DNA concentration was about
10 g/L. This 3,5-DNA concentration was higher
10.0% (v/v). One subsample at each acetonitrile
concentration was acidified to pH 3.5 with acetic
than that determined for the unpreserved sample
30
pH 2.0
3.5
20
10
Unacidified
0
20
40
Storage Time (days)
Figure 1. Stability of TNB in samples acidified to pH2, pH 3.5, and left
unacidified.
5




 Previous Page
Previous Page
