16
a manner similar to that discussed
above, except the analytes tested
were tetryl, 2ADNT, 4ADNT, and
12
3,5-DNA, an analyte recommended
Unacidified
for inclusion in Method 8330 (Walsh
Control
et al. 1993). Tetryl was chosen be-
8
pH 3.5
cause it had been demonstrated to
be unstable with regard to both re-
duction and hydrolysis when held
4
in a soil matrix for very short peri-
ods (Jenkins 1994). The three amino
pH 2.0
compounds were selected because
0
20
40
of the potential for protenation at
Storage Time (days)
the low pH used in two of the pres-
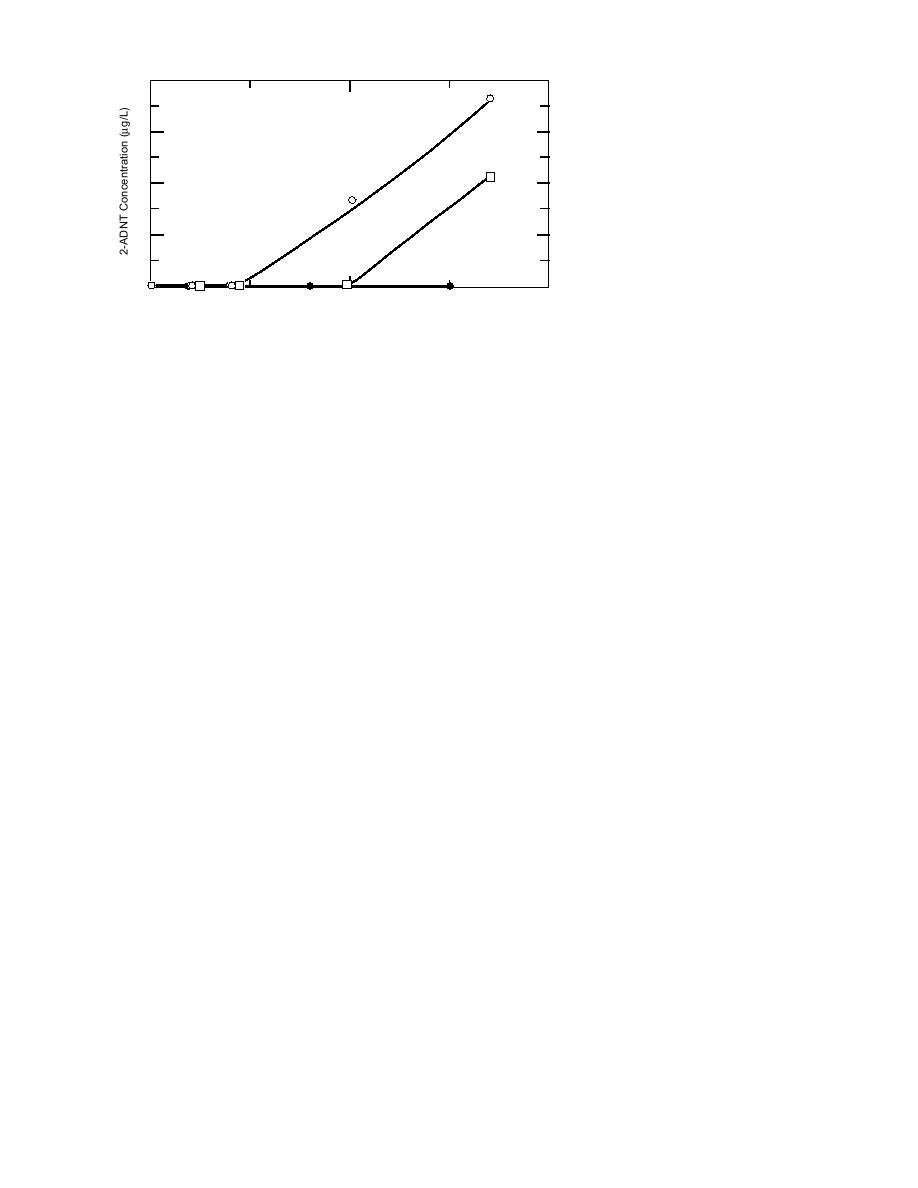
Figure 6. Production of 2ADNT in samples acidified to pH 2, pH 3.5, or ervation techniques, which could
left unacidified.
affect their long-term stability.
The results for tetryl were similar
68%. Acidification to pH 3.5 with acetic acid re-
to that reported earlier for TNB. The concentra-
sulted in increased stability of TNT relative to TNB
tion of tetryl rapidly declined in the unpreserved
with the concentration at day 34 of 32.6 g/L (Fig.
control such that after seven days only about 50%
5). No loss of TNT was observed for the sample
remained (Fig. 7). The loss of tetryl was accompa-
acidified to pH 2 with sodium bisulfate over the
nied by the production of a transformation prod-
entire 30-day storage period. As with TNB, the
uct that eluted about 1.6 min prior to tetryl (4.6
loss of TNT was accompanied by the production
min vs. 6.2 min for tetryl). This transformation
of the monoamino transformation products (Fig.
product was noted in the tetryl soil holding-time
6). The effects of storage with various levels of
study discussed above, but the compound was
ACN, with and without acidification to pH 3.5,
not identified. Acidification to pH 2 and acidifica-
were similar to that described above for TNB.
tion to pH 3.5 with an acetonitrile concentration
Overall, the three storage conditions that were
of 2.5% were very successful in stabilizing tetryl
successful in preserving TNB were also successful
over the entire 31-day study. Stabilization using
in preserving TNT. As discussed above, RDX was
an acetonitrile concentration of 7.5% without acidi-
stable in the control sample and was unaffected
fication appeared to be a slightly less effective pre-
by any of the chemical preservatives tested.
servative, although no transformation products
Thus, of the various stabilization techniques
were observed even after 31 days of storage. The
investigated in this initial study, three appeared to
small differences in concentration for the three
be quite successful:
preservatives shown in Figure 7 could be a result
(1) acidification to pH 2 with sodium bisul-
of poor quantitation due to the development of a
fate,
noisy baseline as the samples aged. This appears
(2) acidification to pH 3.5 with addition of ACN
to be due to long-term storage of samples contain-
to a concentration of 2.5% or greater, and
ing acetonitrile.
(3) addition of ACN without acidification to
The results for 3,5-DNA were quite different
achieve a concentration of 7.5% or greater.
from the results with tetryl. For the unpreserved
In all cases examined, TNB, TNT, and RDX were
control, the concentration only declined from 57
g/L to 41 g/L after 31 days. Concentrations of
stable for at least 30 days when samples were pre-
served using these three techniques. Without
3,5-DNA in the samples stabilized using the three
preservation, TNB and TNT were unstable in these
different preservatives did not appear to be sub-
matrices.
stantially different from one another. For the
2ADNT and 4ADNT, no apparent losses of these
two compounds were observed with the unpre-
Further evaluation of successful
served control sample held for 31 days. There ap-
methods of preservation
To further evaluate these options, the stability
peared to be a slightly lower recovery of both com-
of several other SW846 8330 target analytes were
pounds for the sample preserved at pH 2 using
evaluated over a 31-day period. The test was con-
sodium bisulfate. This result was obtained using
ducted using fortified Connecticut River water in
the direct-injection RP-HPLC method without neu-
8




 Previous Page
Previous Page
