The rate of water movement to the freezing
important phenomenon in all its complexity, cou-
front; and
pled with judiciously selected empirical lab and
The freezing process.
field studies, will ultimately lead to the ability to
Salts are excluded in the freezing process, freez-
accurately predict frost heaving.
ing points are depressed by salts, and unfrozen
water contents are increased in the presence of
EFFECTS OF FREEZING AND THAWING
salts. (See previous discussion on Soil moisture.)
ON SOIL CHEMICAL PROPERTIES
These factors would reduce the water available for
Freezethaw processes and low temperatures
forming segregated ice. Additionally, lower tem-
influence chemical reactions, chemical transport
peratures reduce the soil hydraulic conductivity
and nutrient availability in soils. These interac-
(Perfect et al. 1991). Salts in freezing soils reduce
tions, in turn, influence mineral weathering rates,
the capillary rise and soil hydraulic conductivity
pedogenesis, contaminant remediation and reveg-
(Kay and Scott 1973, Sheeran and Yong 1975,
etation of disturbed lands in cold regions.
Chamberlain 1983, Cary 1987). These factors that
reduce water flow to the freezing zone are proba-
bly the most significant factors explaining lower
Chemical reactions
Two of the most important chemical reactions
frost heaving in the presence of salts. The frozen
affected by freezing and thawing are precipitation
fringe generally thickens in the presence of salts
dissolution and cation exchange. Solute exclusion
(Sheeran and Yong 1975, Yong and Sheeran 1978,
during ice formation leads to supersaturated solu-
Chamberlain 1983), which could reduce the wa-
tions, which promotes the precipitation of second-
ter flux to segregated ice lenses.
ary minerals in soil, alters solution-phase
Solutes clearly affect the lensing process (Shee-
compositions (which may promote the dissolution
ran and Yong 1975, Chamberlain 1983). Chamber-
of primary minerals) and shifts equilibrium to-
lain (1983) found plentiful but thin ice lenses
ward increased mineral weathering (Fig. 4) (Hal-
forming in the presence of salts. This type of ice
let 1978, Zvereva 1982, Sletten 1988, Richardson
lens formation could reduce the flow of water in
et al. 1990). Martynenko et al. (1992) subjected
the frozen fringe, where segregated ice forms. Salts
ground primary minerals in oxalic acid solutions
can interact with soil particles, causing them to
to 70 freezing cycles. The interaction of acidic hy-
either aggregate or disperse, depending on solute
drolysis and cryogenic comminution enhanced
type and concentration (Lambe et al. 1971, Sposi-
mineral grain fragmentation and chemical weath-
to 1989); this, in turn, will affect soil permeability
ering.
and the resultant water flux.
Salts may also affect the depth of frost penetra-
tion. The deeper the zone of freezing, the more
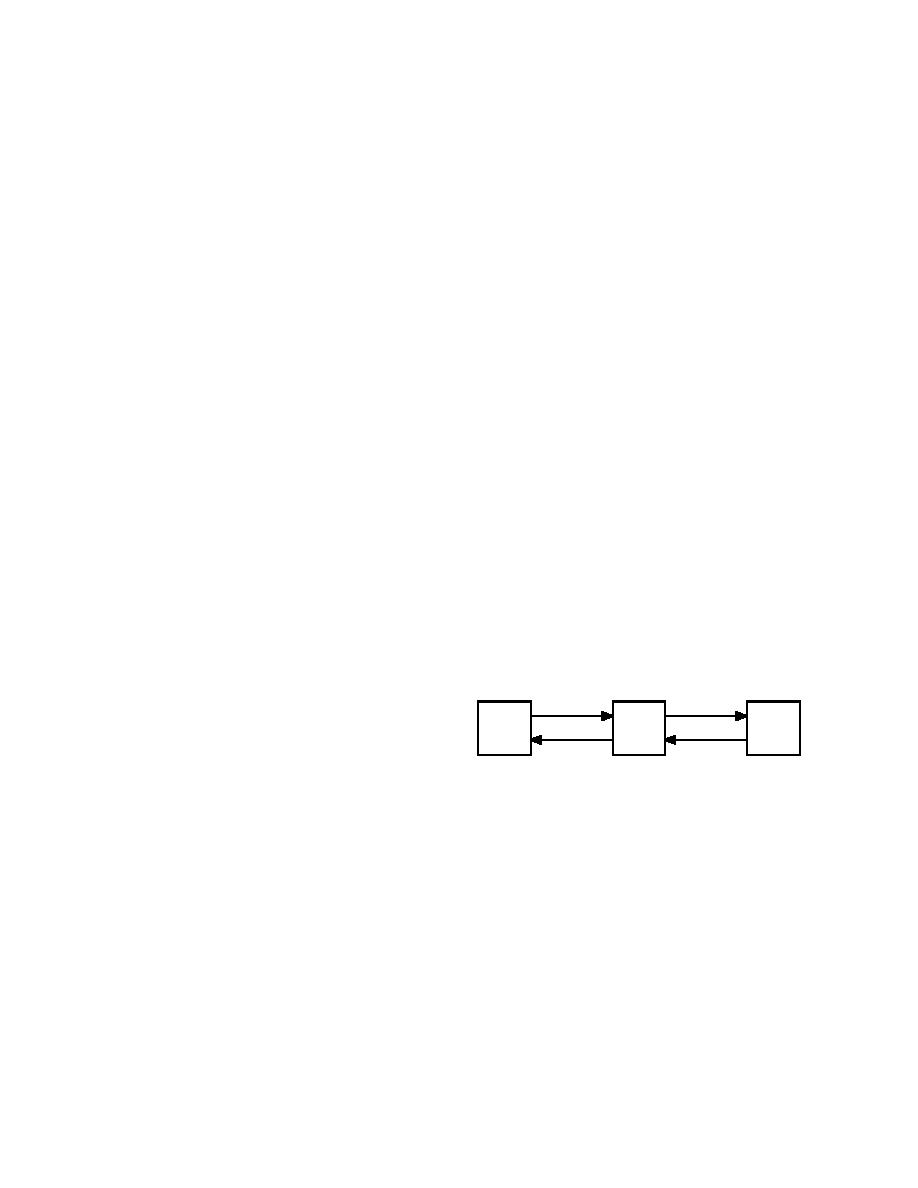
Precipitation
Exchange
Soluble
Soil
important overburden pressure becomes in limit-
Ion Exchange
Complex
Ions
Minerals
ing segregated ice formation. Sheeran and Yong
Dissolution
(1975) found that the depth to the freezing front
Figure 4. Ion exchange equilibria in a soil system.
decreased with increasing salt concentration. On
The precipitation of silica in the lower horizons
the other hand, Mahar et al. (1983) found that the
of soil profiles has been attributed to freezing
rate of advance of the freezing front increased with
(Slavnyy and Vorob'yeva 1962). The distribution
increasing salinity; they attributed this to the grad-
of Na and Mg sulfate minerals in soils is tempera-
ual release of the latent heat of fusion over a range
ture dependent, with low temperatures favoring
of temperatures and depths due to changing sol-
the precipitation of Na sulfates (e.g., mirabilite:
ute concentrations. Cary (1987), using a simula-
Na2SO4.10H2O) relative to Mg sulfates (Arndt and
tion model, predicted a greater depth of frost
Richardson 1989, Richardson et al. 1990); in these
penetration with increased salt concentration.
papers it was hypothesized that freezing from the
Contradictions abound in the literature on frost
top down may concentrate the more soluble Mg
heaving, probably because of the complex inter-
sulfates at greater depth, leading to the develop-
actions among the driving forces controlling this
ment of surficial sodic (Na) horizons.
process. Frost heaving is clearly a complex pro-
Freezing of Ca and Mg bicarbonate solutions
cess dependent on soil properties, moisture con-
leads to the precipitation of the more insoluble
ditions, solute concentrations and energy balance.
CaCO3, thereby increasing the solution-phase
Such complexity can not be explained by simple
Mg2+/Ca2+ ratio (Vlasov and Pavlova 1969, Ivanov
models. Only a continuing effort to model this
7




 Previous Page
Previous Page
