higher plants may obtain a significant proportion
and chemical processes. Significant CO2 emissions
of their annual nutrients during the fall as the re-
from wet tundra soils extended through February
sult of increased soluble sugars, phosphorus and
March in some northern sites (Federov-Davydov
1993, Zimov et al. 1993). On the other hand, in oth-
amino acids in solution.
er soils, CO2 fluxes went to zero when soils froze
The effect of freezethaw processes on nutrient
(Federov-Davydov 1993, Zolotareva and Demkina
availability is a critical factor in revegetation of
1993). Zimov et al. (1993) hypothesized that ener-
severely disturbed lands in cold regions. Because
gy production by microbes was an important con-
nitrogen (N) is the element that most frequently
tributor to soil heat balance, preventing the freezing
limits terrestrial plant growth (Raven et al. 1986),
of the entire soil profile, which allows significant
it has been the most intensively studied nutrient
microbial activity to occur even during the winter.
in cold regions.
Because of the high temporal and spatial variabili-
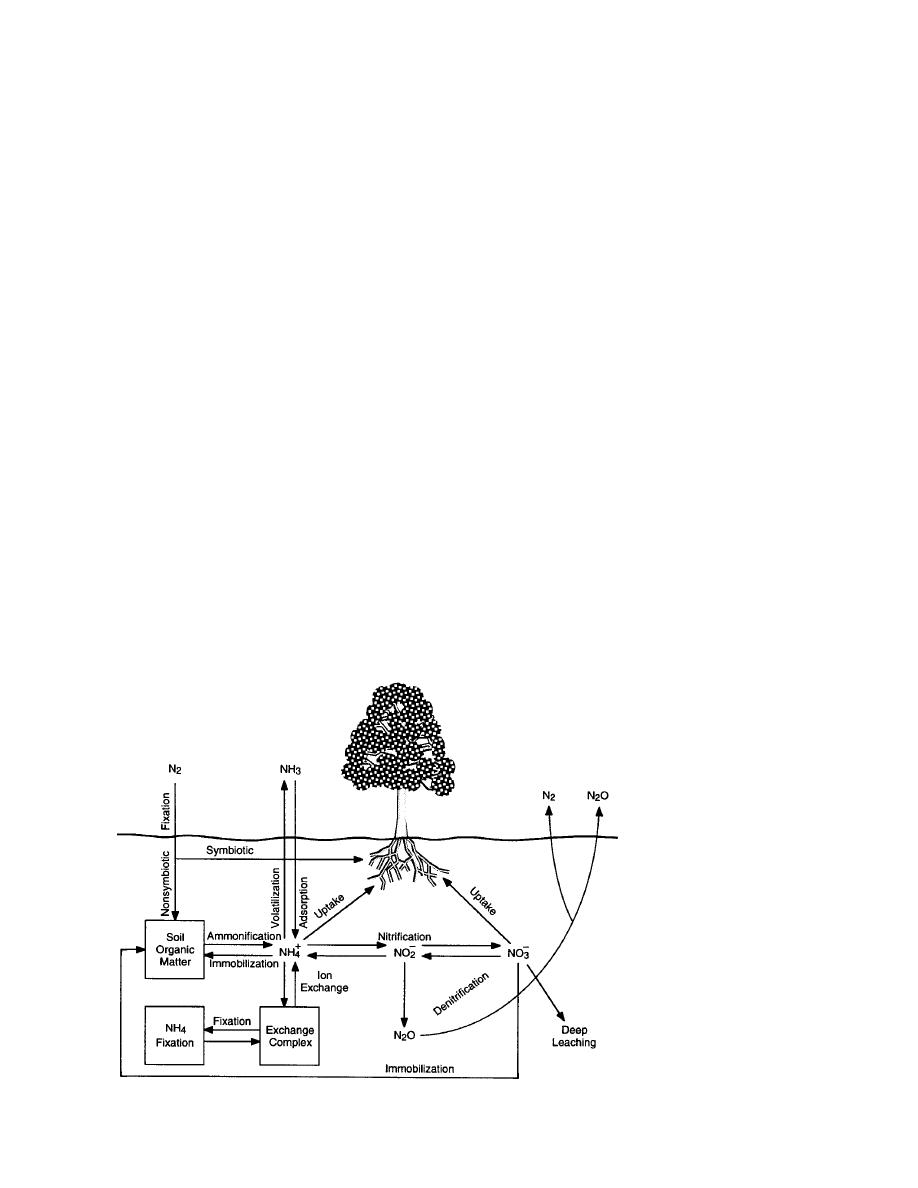
The dominant processes controlling the cycling
ty in winter gas fluxes (Federov-Davydov 1993,
of terrestrial N (Fig. 5) include:
Zimov et al. 1993), their overall significance for the
N2 fixation, which directly converts atmo-
global carbon balance is unclear at present.
spheric N2 gas into organic N via N-fixing
Extracellular enzyme activities (urease, phos-
bacteria;
phatase, sulfatase) have been detected in soils at
Ammonification, which converts organic N
temperatures as low as 20C (Bremner and Zan-
into ammonium ( NH+ );
4
Nitrification, which converts NH+ into nitrate
uta 1975); these low-temperature activities are be-
4
( NO3 ) through the nitrite ( NO2 ) intermedi-
lieved to be occurring in the unfrozen water at
surfaces of soil particles. Freezethaw events in
ary;
Cation-exchange reactions between NH+ and
tundra water tracks in the fall can cause rapid de-
4
creases in soil redox potentials; associated with
exchange complexes;
NH+ fixation, which can render NH+ diffi-
these changes are concomitant increases in extra-
4
4
cellular enzyme activity (cellulases, phosphomo-
cultly exchangeable;
Plant uptake of NH+ and NO3 ;
noesterase, proteases) (Linkins 1987). Enzyme
4
Immobilization of NH+ and NO3 by microb-
activity continues to increase over three or four
4
freezethaw cycles. It appears that the number of
ial processes;
freezethaw events, rather than the duration of any
Denitrification, which converts NO3 into gas-
one event, is the principal factor determining total
enzyme activity. Linkins (1987) hypothesized that
Ammonia (NH3) volatilization, which con-
verts NH+ into NH3 gas;
4
Adsorption of NH3 gas by soils; and
Leaching, which is a particular problem for
the negatively charged NO3 ion (Clark and
Rosswall 1981, Marion 1987).
Figure 5. Nitrogen cycle in a terres-
trial ecosystem. (After Marion 1987.)
11




 Previous Page
Previous Page
