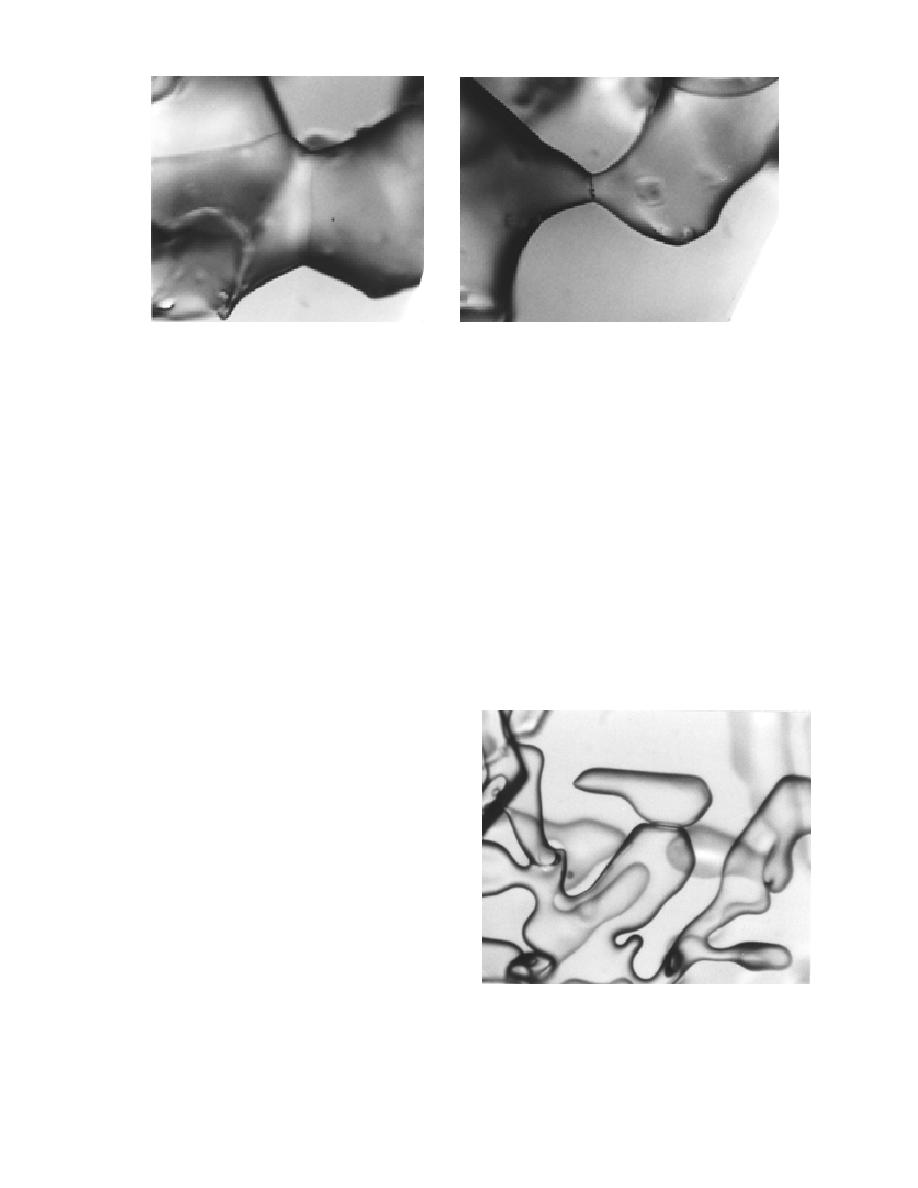
Figure 7. Snow grains stored at 24F for several years showing distinct grain-boundary grooves at the
bonds.
leading in this regard since they where made from
period of the formation of a bond when surface
sections and not from the whole grains. As dis-
diffusion, or even pressure meltingregelation,
cussed later, it is important to know if the dihe-
could occur. In fact, it makes a lot of sense to think
dral angle of about 145 is always maintained
about different mechanisms dominating during
throughout sintering, or if the angle is small ini-
different phases of bond growth, just as Alley et
tially and then increases as sintering proceeds.
al. (1982) and Wilkinson (1988) did for densifica-
This question should be answered with more mi-
tion. Ideas based on a concave curvature (Fig. 4)
croscopic observations, since its answer will de-
could dominate during the early phases when
termine how we think about the processes and
sintering is most rapid, and microphotographs
how they are modeled. For example, if an angle of
are not yet available to disprove the notion of a
about 145 is always maintained, there will neces-
simple concave bond. Figure 6 shows that one
sarily be concave curvature adjacent to the grain-
grain can be purely convex while the other has
boundary groove, at least during the initial stage
mixed curvature, and thus different processes may
of sintering. This will affect all of the processes,
operate on adjacent grains, or even on different
regardless of what they are.
parts of one grain. Figure 7 shows the nature of
grain bonds in snow stored at a low temperature
for several years: these bonds are crystal bound-
aries with grain-boundary grooves and the ap-
propriate grain-boundary groove angle for ice and
water vapor, about 145 2 (Ketcham and Hobbs
1969). Because the grains are not packed in a regu-
lar manner, part of the grain surface can be still be
concave, even at this late stage of sintering.
The formation of grain-boundary grooves is
not limited to old snow that has had a long time to
reach its equilibrium form. A fresh snow bond of
a similar nature is shown in Figure 8. In fresh
snow, the grain-boundary groove angle appears
to be much less than the equilibrium value of 145
because the bonds have just formed. The bond
grows rapidly at first as the stress imbalance at
the junction is reduced, probably with an expo-
nential decay. Kuroiwa's (1962) photographs and
Figure 8. Fresh, dry snow with newly formed bonds
my own microscopic observations suggest to me
showing a grain boundary with a grain-boundary
that the dihedral angle increases with time of
groove instead of reverse curvature.
sintering. However, his photographs can be mis-
6




 Previous Page
Previous Page
