which leads to
BC = νB B + νC C + νB RT ln(mB γ B ) + νCRT ln(mC γ C )
O
O
(38)
and
(
)
1/(νB + νC )
BC = BC + (νB + νC ) RT lnmBC νCB vBC
γ BC .
ν
ν
O
(39)
By
OC = vC O + vB O
(40)
B
C
B
the mean-ionic activity coefficient is therefore defined as
1
γ BC d=f γ BB γ CC (vB + vC ) .
vv
e
(41)
12
8
4
0
4
40
20
0
20
40
60
60
Temperature (C)
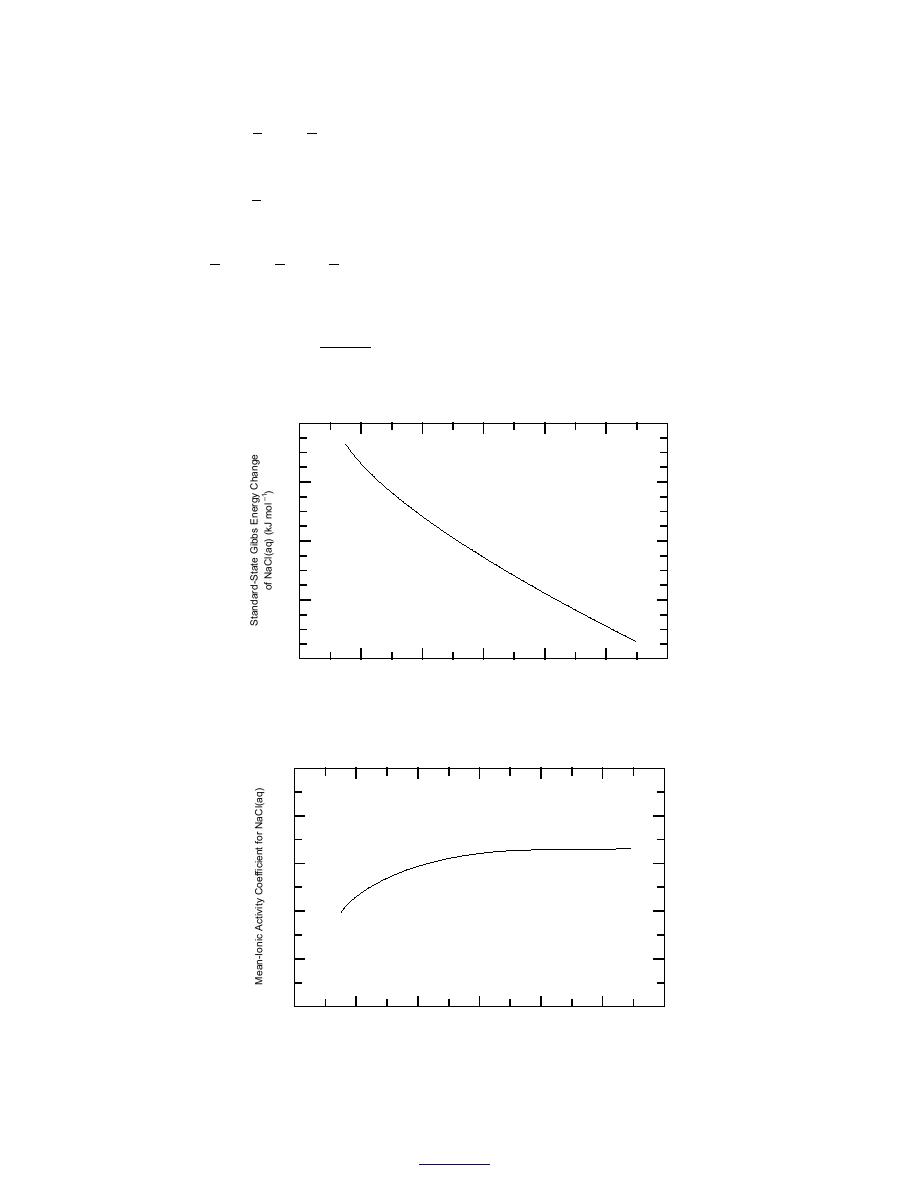
Figure 8. Standard-state chemical potential of NaCl(aq) as function of
temperature.
1.0
0.8
0.6
0.4
0.2
0
60
60
40
20
0
20
40
Temperature (C)
Figure 9. Mean-ionic activity coefficient of NaCl(aq) (p = 0.1 MPa,
m = 1.0 mol kg1) as function of temperature.
14
TO CONTENTS




 Previous Page
Previous Page
