plateau around 50% water. The sharp rise in re-
was more intensely yellow than the Alumina-A
covery from low water contents to 50% indicated
since all the picrate was sorbed on the top surface,
that extracts of field-moist soils would produce
as opposed to being distributed within the sorbant
unpredictable results if not diluted with water up
bed of the cartridge. The Alumina-A did not re-
to the 50% plateau.
tain picrate from water only, whereas the Anion
The field methods for TNT, 2,4DNT and RDX
membrane showed its greatest retention from
specify the use of 100 mL of acetone to extract 20 g
water.
of soil. These methods use about 60 mL of the
Both sorbants that retain picrate are ion-ex-
common extract for analyses. This leaves 40 mL
change materials; therefore, elution and quantifi-
for the picrate assay. Since it is difficult to remove
cation of the picrate is feasible. For the Anion disk,
all of the remaining acetone from the extraction
10% concentrated sulfuric acid/methanol (v/v),
bottle, 30 mL of acetone extract diluted one-to-one
and for the Alumina-A cartridge 2% concentrated
with water was chosen for subsequent tests.
sulfuric acid/acetone (v/v), were the mildest con-
Further increases in recovery were realized by
ditions that resulted in elution. Lower concentra-
optimizing extraction and elution conditions.
tions of acids, different acids or different solvents
Breakthrough tests were conducted at different ex-
were not successful. Under conditions of such high
acidity, the picrate is converted to undissociated
traction rates using 60 mL of a 50% aqueousac-
picric acid and is colorless, as it is in MeCl2. The
etone solution fortified with picric acid. At a flow
rate of 10 mL/min, about 4% of the picrate was
eluted solution is filtered through a Millex SR
not retained. The loss increased from 4% to 13%
syringe filter placed on the tip of the cartridge,
when the flow rate was increased to 40 mL/min.
then diluted with water until the pH was raised
Elution with a 10-mL aliquot of 2% H2SO4acetone
above the pKa of picric acid, resulting in the for-
at a flow rate of 5 mL/min was sufficient to re-
mation of the colored picrate anion. This enabled
cover 8891% of the retained picrate.
both quantification using a field-portable spectro-
To achieve the brightest color for quantifica-
photometer and confirmation by the unique color-
tion, as much acetone as possible should be used
changing behavior of picric acid to picrate (Deniges
with the minimum water to drive the ionization
1923). The eluent from the Empore Anion mem-
and subsequent color change. The absorbance of
brane did not require additional filtration.
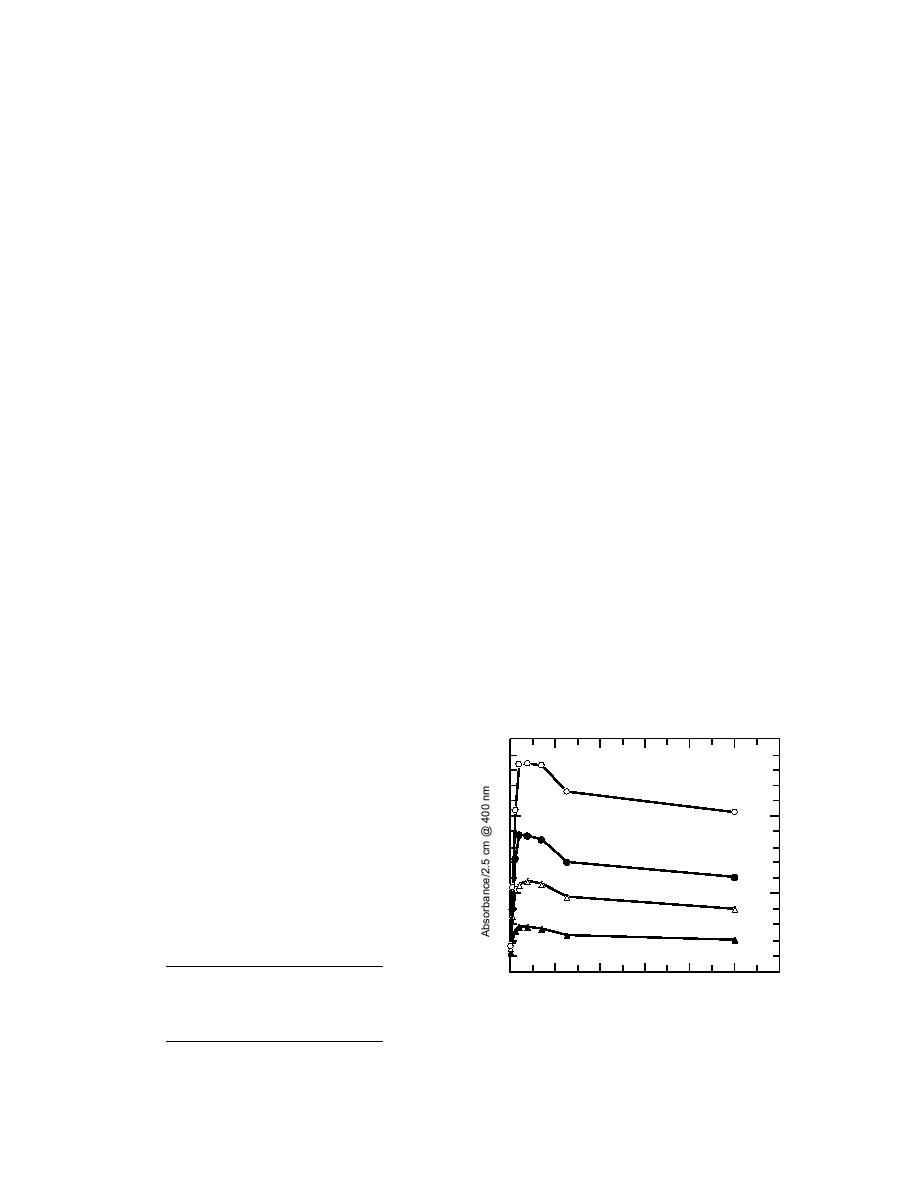
picrate in acetone is highly dependent on the wa-
ter content (Fig. 5). Once sufficient water is present,
Optimization of conditions
additional water only dilutes the sample and re-
Although the Empore Anion membrane did
duces the color. However, the amount of water
retain picrate from both acetone and aqueous ex-
that gives the highest absorbance is in a very nar-
tracts, it was not chosen for the soil method be-
row peak of the curve. Precise measurement
cause one membrane costs nearly ten times as
would then be critical to duplicate the predicted
much as one Alumina-A cartridge.
Acetone extracts of field-moist soils will con-
tain variable amounts of water. Since the initial
1.5
retention experiments showed that the Alumina-
A cartridge did not retain picrate from water alone,
an additional test was run to determine what per-
10 g/mL
centage of water in acetone would result in the
1.0
maximum retention of picrate. The results are
listed in Table 1. The maximum occurred at a broad
6 g/mL
4 g/mL
Table 1. Recovery of 4.8 g/mL pi-
0.5
crate from wateracetone mixtures.
2 g/mL
Wateracetone
Recovery of picrate
(%)
(%)
0
20
40
60
80
100
120
8
4
Percent Water
25
38
Figure 5. Dependence of picrate absorbance on wa-
50
86
75
85
ter content of acetone.
5




 Previous Page
Previous Page
