Development of a Field Method for Quantifying
Ammonium Picrate and Picric Acid in Soil and Water
PHILIP G. THORNE AND THOMAS F. JENKINS
with picrate remaining in solution. Mixtures of
INTRODUCTION
sodium ions with calcium clays or calcium ions
with sodium clays produced intermediate effects.
Source, fate and toxicology of
These experimental studies suggest that transport
ammonium picrate and picric acid
Ammonium picrate (ammonium 2,4,6-trinitro-
of picrate in the environment will be highly vari-
phenoxide, CAS 131-74-8)--Explosive D--was
able, depending on the organic and mineral com-
used in armor-piercing shells, bombs and rocket
position of each soil. Kayser and Burlinson (1988)
warheads by the U.S. military from the turn of the
found that picrate migrated through four soils in
century to after World War II. It is no longer manu-
lysimeter studies. Van Denburgh (reported in
factured but now represents 8% of the demilitari-
Layton et al. 1987) found that picrate had migrated
zation inventory. Picric acid (2,4,6-trinitrophenol,
from a disposal bed at an ammunition plant.
CAS 88-89-1) was used as a grenade and mine
Ruchholt and Norris (1946) reported finding one
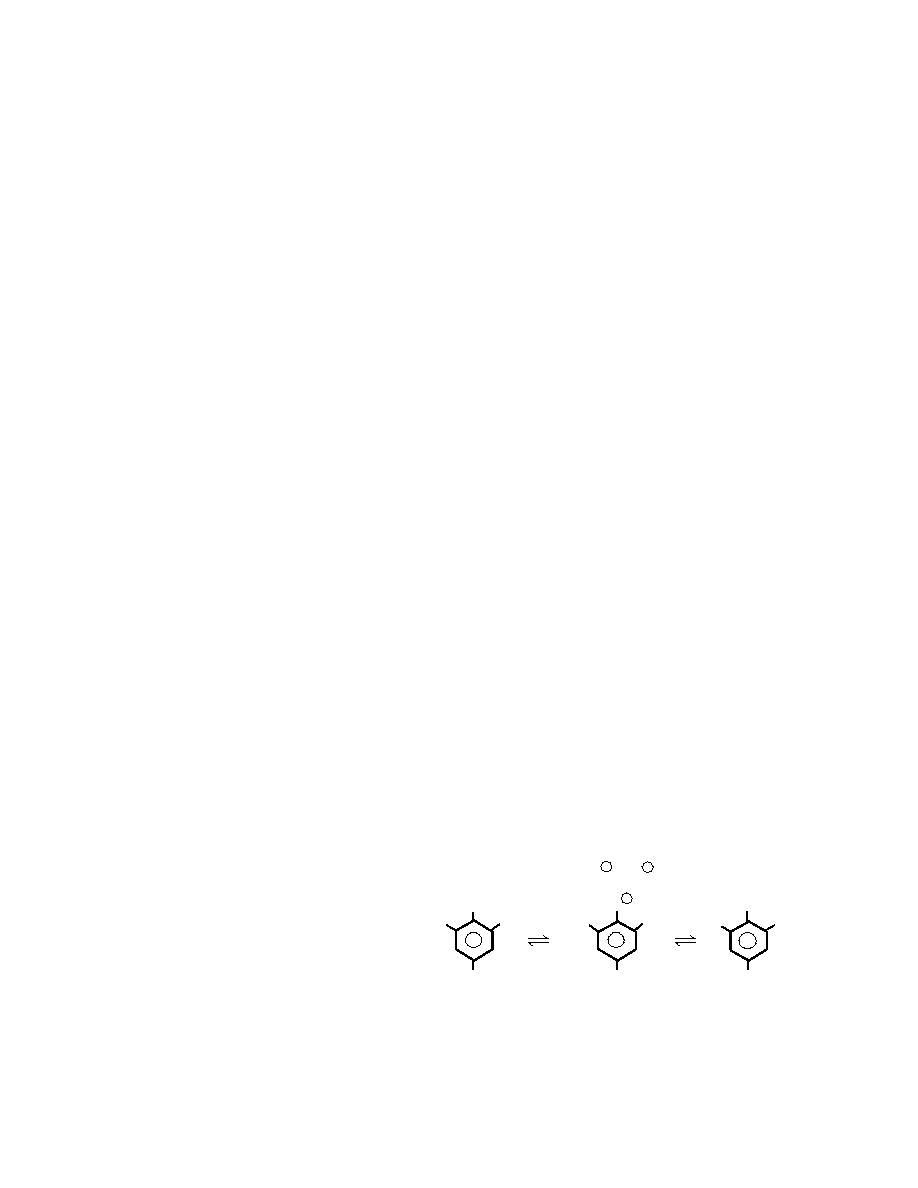
filling (Meyer 1987) (Fig. 1).
soil that retained "appreciable" amounts of picrate.
Layton et al. (1987) reported comprehensively
Most of the toxicological work reported by
on the production, toxicology and environmental
Layton et al. (1987) was on skin adsorption and
fate of ammonium picrate and its parent com-
inhalation of ammonium picrate dust. Few data
pound, picric acid. When dissolved in water, both
were available on the chronic effects of ingestion
ammonium picrate and picric acid dissociate to
of picrate. The most recent research (Wyman et al.
the picrate ion. Aqueous solubilities for both com-
1992) deals with lethal-dose determinations. The
pounds are over 10 g/L, and they appear to present
EPA has not set an action level for ammonium
an extremely mobile environmental contaminant.
picrate or picric acid in soil or water. Layton et al.
Goodfellow et al. (1983) showed that the parti-
(1987) estimated an Allowable Daily Intake (ADI)
of 137 g/kg-d. Since the estimated ADI is simi-
tioning of picrate from estuarine water to organic
sediment was very low. This follows from the low
lar to other secondary explosives, similar field de-
octanolwater partition coefficient for picric acid
tection limits were sought in this research (i.e.,
low g/g in soil and low g/L in water). There is
(log Kow = 1.6) (Layton et al. 1987).
On the other hand, Layton et al. (1987) predicted
potential for picric acid to be transformed to
that picrate will act like phenolic pesticides
and become incorporated into or bound to
+
+
+
+
H or NH4
or
4
humic substances. Chang and Anderson
(1968) studied the flocculation of clays by
OH
OH
O
ONH 4
picric acid and found that the degree of floc- O 2 N
NO 2
O2 N
O 2N
NO 2
NO 2
culation depended on the nature of the clay
and associated ions. When picric acid was
mixed with solutions containing calcium ion
NO 2
NO 2
NO 2
and calcium clays, flocculation occurred rap-
Picric Acid
Ammonium Picrate
Picrate Ion
idly and completely, removing picrate from
solution. Mixtures containing sodium ions Figure 1. Chemical structures of picric acid, picrate ion and
and sodium clays formed stable suspensions, ammonium picrate.




 Previous Page
Previous Page
