100
1.2
Water
98
1.0
Overnight Sonic Extraction
96% Acetone
Acetone
96
0.8
Water
94
0.6
92
0.4
90
0.2
88
0
20
40
60
80
100
120
0
10
20
30
Picrate (g/mL)
Extractant (mL)
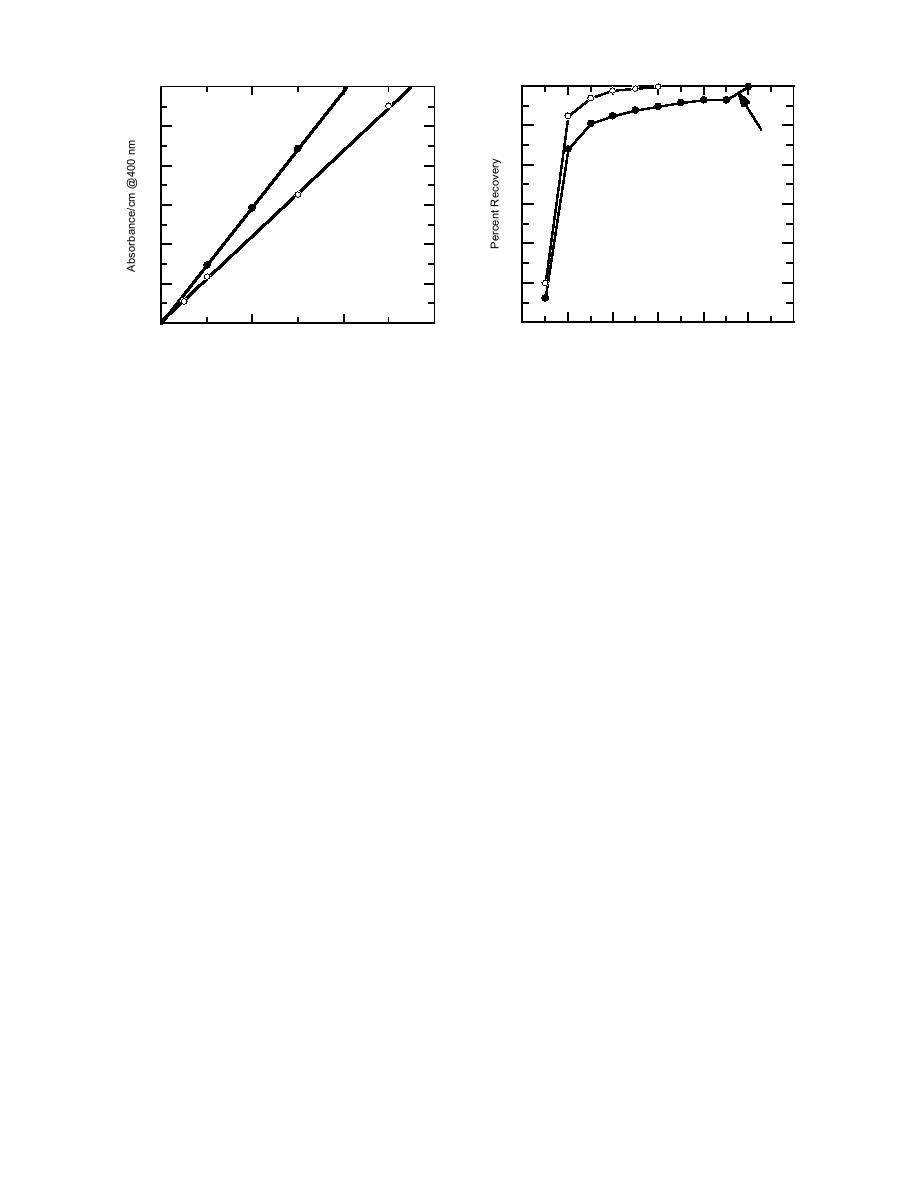
Figure 3. Absorbances for picrate in acetone and
Figure 4. Recoveries of picrate from Hawthorne soils
water.
by sequential 10-mL extractions.
was any fresh plant material or humus present.
was placed in a 22-mL glass vial with 10 mL of
This problem could be overcome with larger vol-
extractant and shaken manually for three minutes.
umes of solvent, but this seemed environmentally
The vials were then centrifuged for three minutes
unwise. Since MeCl2 is denser than water, it col-
and the extractant decanted and filtered through
a 0.45-m Millex SR syringe filter. The quantity of
lects at the bottom of extraction vessels, dictating
the use of separatory funnels. These are expensive
picrate extracted was determined spectrophoto-
and difficult to use and clean in the field. Several
metrically. Further aliquots (10 mL) of extractant
other less-toxic solvents with densities less than
were added to the soil and the procedure repeated
water were tried to see if they would extract picric
until no more yellow color was extracted. Since
acid and exchange it onto Florisil. Isooctane pro-
the acetone extractions appeared to reach a pla-
duced a clear extract that, when picric acid was
teau, the vial containing the tenth aliquot was
placed in a sonic bath overnight. This process re-
present, turned Florisil yellow, but the extraction
moved the remaining picrate. Recoveries of picrate
efficiency of picric acid from a field-contaminated
from this soil were calculated as a normalized per-
soil was very low. Ethyl ether was an efficient ex-
tractant but resulted in a yellow extract, since it
centage based on the total amount recovered from
also dissolves some aqueous picrate ions. No ex-
each sample. The percent recoveries in the first
change with Florisil was apparent. No further ex-
10-mL aliquot were 89% for acetone and 90% for
periments were conducted using nonpolar sol-
water (Fig. 4).
vents and Florisil.
Several polar solvents (acetone, methanol, iso-
Selection of ion exchange materials and
propanol, acetonitrile and water) were used to
binding and elution conditions
extract a soil from Hawthorne AAP known to be
Two ion-exchange materials were chosen for
contaminated with picric acid. All extracts were
investigation. Alumina-A solid-phase extraction
yellow. By far the brightest yellows resulted from
cartridges are used in the RDX field method
the acetone and water extractions. This soil had a
(Jenkins and Walsh 1992). Empore Anion extrac-
moisture content of 4%, so standard curves for
tion membranes were selected because they have
picrate in 96% acetone/4% water and 100% water
an allowable flow rate approximately 20 times
were constructed and showed a linear relation-
greater than the extraction cartridges and would
ship between absorbance at 400 nm and concen-
provide a different exchange chemistry. Solutions
containing 1 g/mL of picric acid in acetone or
tration (Fig. 3). The visual detection threshold for
water were passed over Empore Anion membranes
picrate in an acetone or water extract corresponds
to a soil contamination value of approximately 5
and Alumina-A cartridges. For the acetone solu-
g/g. The extraction efficiencies of acetone and
tions, both sorbants became yellow, indicating the
retention of picrate ions. The Anion membrane
water were then determined. A 2-g sample of soil
4




 Previous Page
Previous Page
