ratory in ice-filled coolers (FedEx overnight). Upon
phenylmethyl-polysiloxane (RTX-225 from Restek).
arrival at CRREL the samples were frozen at 30C
Further details of the procedure may be found in SW-
until extraction and analysis within two weeks.
846 Method 8095 (draft) (USEPA 2000b). If analyte con-
centrations were within the linear range of the ECD,
Soil extraction
concentrations reported were taken from the determin-
For extraction, the jars containing the soil samples
ation on the primary column, unless there appeared to
were moved to the laboratory and allowed to warm to
be co-elution with another compound. In such cases,
room temperature. Samples were homogenized by
reported concentrations were taken from the determin-
removing small stones, breaking up the material in the
ation of the confirmation column. Detection limits for
the GC-ECD analysis were about 1 g/kg for di- and
sample jar using a spatula, and stirring the contents thor-
trinitroaromatics, and 3 g/kg for RDX (Table 3).
oughly. The sample sometimes consisted of just soil
but usually was a combination of both soil and organic
matter. A 2.00-g portion of undried material was then
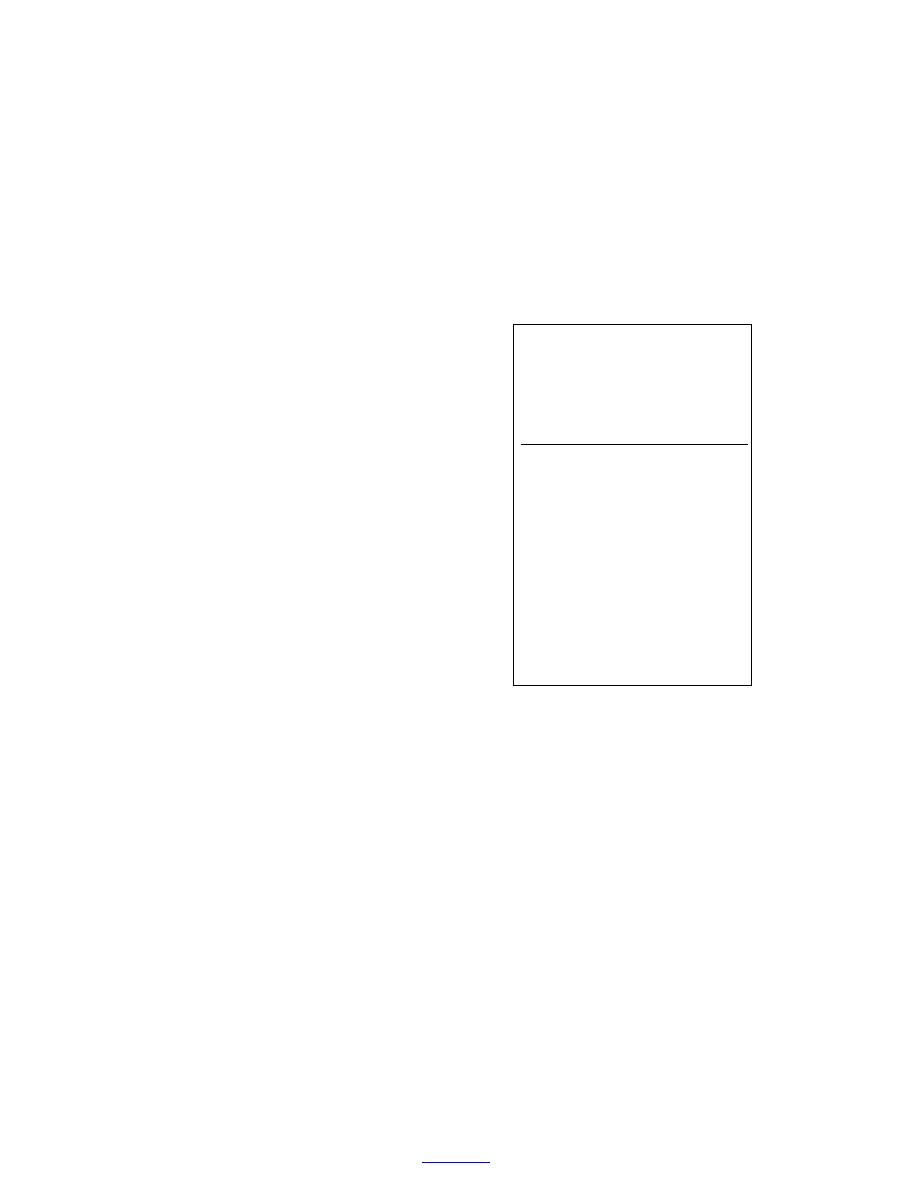
Table 3. Method detection limits (g
removed from the jar in several increments and placed
kg1) of nitroaromatics, nitramines,
in a 22-mL scintillation vial. A 5.00- or 10.0-mL aliquot
and nitrate esters in soil determined
by GC-ECD (Walsh and Ranney
of AcN was added to each sample, depending on the
1999).
amount of organic matter present. The vials were then
placed on a vortex mixer for 30 seconds to suspend the
MDL
(g kg1)
Analyte
soil particles, and the vials were placed in an ultrasonic
bath for 18 hours. The temperature of the bath was
1,3-Dinitrobenzene
0.8
maintained at less than 25C with cooling water. The
2,6-Dinitrotoluene
0.8
2,4-Dinitrotoluene
0.8
vials were then removed from the bath and allowed to
1,3,5-Trinitrobenzene
3
stand undisturbed for 30 minutes. A 2.5-mL aliquot of
2,4,6-Trinitrotoluene
1
each extract was removed using a glass syringe and
RDX
3
filtered through a 25-mm Millex-FH (0.45-m) dispos-
4-Amino-2,6-dinitrotoluene
1.5
2-Amino-2,4-dinitrotoluene
2.5
able filter, discarding the first milliliter and collecting
Tetryl
20
the remainder in a clean autosampler vial. The extracts
HMX
25
were kept cold prior to and during analysis.
3,5-Dinitroaniline
2
Nitroglycerin
20
PETN
25
Soil extract analysis
o-Nitrotoluene
15
The vials containing the AcN (acetonitrile) soil
m-Nitrotoluene
12
extracts were placed into GC autosampler trays that
p-Nitrotoluene
10
were continuously refrigerated by circulating 0C gly-
col/water through the trays. The extracts were analyzed
Extracts were also analyzed by RP-HPLC according
by gas chromatography using a micro-electron capture
to SW-846 Method 8330 (EPA 1994). When concentra-
detector (GC-ECD). Results were obtained on a HP-
tions were above 500 g/kg, the reported concentrations
6890 GC equipped with a micro cell Ni63 detector at
were taken from the HPLC analysis, which had a higher
280C according to the general procedure outlined in
range of linearity. The response of the GC-ECD was
inadequate for the reduction products of 2,4-DNT
EPA SW-846 Method 8095 (draft) (EPA 2000b). Direct
injection of 1 L of soil extract was made into a purged
(4A2NT and 2A4NT). Data reported for these analytes
packed inlet port, at 250C, that was equipped with a
were obtained by RP-HPLC. RP-HPLC analysis was
conducted on a modular system composed of a Spectra-
deactivated Restek Uniliner. Primary analysis was con-
ducted on a 6-m- 0.32-mm-ID fused-silica column,
Physics Model SP8800 ternary HPLC pump, a Spectra-
with a 1.5-m film thickness of 5%-(phenyl)-methyl-
Physics Spectra 100 variable wavelength UV detector
set at 254 nm (cell path 1 cm), a Dynatech Model LC241
siloxane (RTX-5 from Restek). The GC oven was
temperature programmed as follows: 100C for two
autosampler equipped with a Rheodyne Model 7125 sam-
minutes, 10C/minute ramp to 260C, two-minute hold.
ple loop injector, and a Hewlett-Packard 3396A digital
integrator set to measure peak heights. Extracts were
The carrier gas was helium at 10 mL/minute (linear
diluted with reagent-grade water (one part extract and
velocity approximately 90 cm/sec). The ECD makeup
four parts water). Separations were conducted on a 15-
gas was nitrogen flowing at 40 mL/minute. If a peak
cm 3.9-mm NovaPak C-8 column (Waters) eluted with
was observed in the retention window for a specific
85/15 water/isopropanol (v/v) at 1.4 mL/minute. Samples
signature compound, the extract was reanalyzed on a
were introduced by overfilling a 100-L sampling loop.
confirmation column, 6-m 0.53-mm ID having a 0.1-
m film thickness of 50% cyanopropylmethyl50%
Concentrations were estimated against multianalyte stan-
9
To contents




 Previous Page
Previous Page
