0.14
dropped below detectable levels (Nam et al.
1994). Thus, P4 is eliminated and does not
0.12
bioaccumulate in the same manner as other
lipophilic toxicants such as PCBs, which con-
0.10
centrate in upper trophic levels.
0.08
Vapor pressure
0.06
The vapor pressure of a liquid or solid is the
pressure of the gas phase in equilibrium with
0.04
the liquid or solid phase at a given temperature
and is used to assess the tendency of a sub-
0.02
stance to evaporate.
The vapor pressure of solid P4 has been
0
8
16
24
32
40
Temperature (C)
measured over the temperature range 23 to
41C and ranged from 0.0004 to 0.13 torr (Dain-
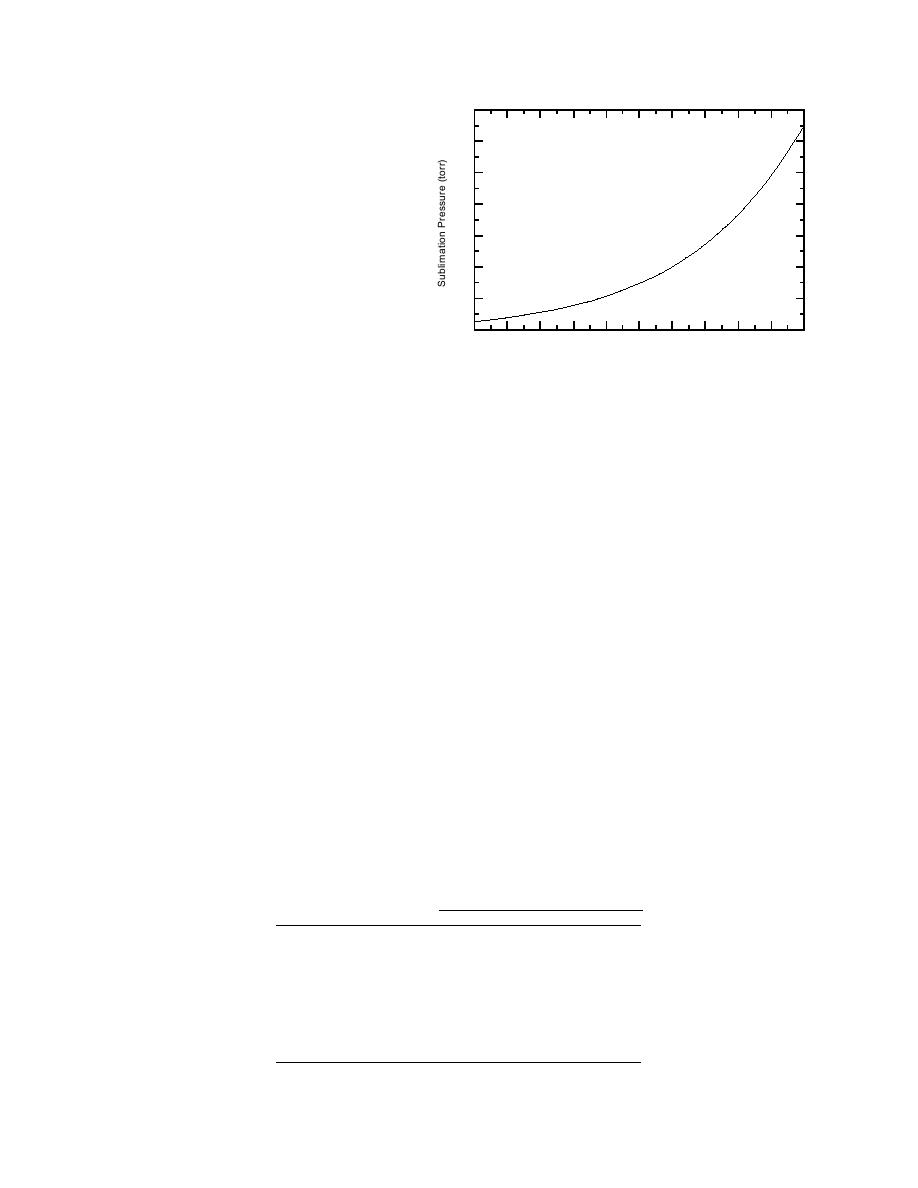
Figure 6. Calculated sublimation pressure for P4.
ton and Kimberley 1950, Nesmeyanov 1963). At
room temperature (2025C) the vapor pres-
sure is 0.0250.04 torr.
through the soil surface (concentrations higher
than 0.01 mg/m3, the threshold limit value, were
Van Wazer (1958) claimed that the following
equation for vapor pressure of solid P4 agrees
measured in a vapor cloud above soil containing
with experimental data:
P4). In the laboratory, 35 mg of P4 as "pellets"
(size not reported) were placed at depths of 2.5
log10P (torr) = 1.9198 (3.084 103)T+
and 10 cm in pots containing 750 g of two types of
soil (acid and calcareous) of approximately 9%
2.7763 log T (2.641 103)/T
moisture. The air above each pot of soil was mon-
(3.258 104)/T2
itored for P4 vapor for three days. The cumulative
(8)
mass of P4 in the air after three days was approx-
which yields a pressure of 0.04 torr at 25C (Fig.
imately 9.5 g for the P4 at 2.5-cm depth and 1.5
and 4.6 g for the acid and calcareous soils with
6).
At 25C the vapor pressure of P4 is on the same
P4 at the 10-cm depth (Table 2). In a second exper-
iment the effect of soil moisture on the amount of
order of magnitude as many semi-volatiles such
P4 vapor escaping from soils containing 35 mg of
as naphthalene. Thus, sublimation could be a sig-
P4 pellets at 5-cm depth was monitored for seven
nificant process in the environment, depending
days. In all cases, P4 was detected in the air above
on temperature. In fact, sublimation of P4 from
the soil (Table 2). There is no clear pattern as to
soil was observed by Warnock (1972) when he in-
the effect of soil type, soil moisture or length of
vestigated the potential use of white phosphorus
time P4 was monitored. Unfortunately soil tem-
as a source of plant-available phosphoric acid.
peratures were only measured intermittently and
During field studies, P4 was observed to escape
Table 2. Mass of P4 recovered as a vapor above soils con-
taining 35 mg of P4 pellets. (After Warnock 1972.)
Soil
Mass (g) of P4 recovered
Soil
Depth
moisture
type
(cm)
(%)
1
2
3
5
6 7 days Total
Acid
2.5
8.6
6.4
2.5
0.5
*
*
*
9.4
Calcareous
2.5
9.3
3.1
4.9
1.6
*
*
*
9.6
Acid
10
8.6
1.1
0.2
0.2
*
*
*
1.5
Calcareous
10
9.3
1.8
1.6
1.1
*
*
*
4.6
Acid
5
4.3
2.3
1.0
0.5
2.2
1.2
0.3
7.5
Acid
5
11.2
2.2
0.6
5.0
0.3
1.7
0.9
10.7
Acid
5
18.1
1.3
2.8
0.5
0.5
0.7
0.2
5.8
Acid
5
31.9
1.8
0.8
0.3
0.6
1.1
0.3
4.7
* Not measured.
7




 Previous Page
Previous Page
