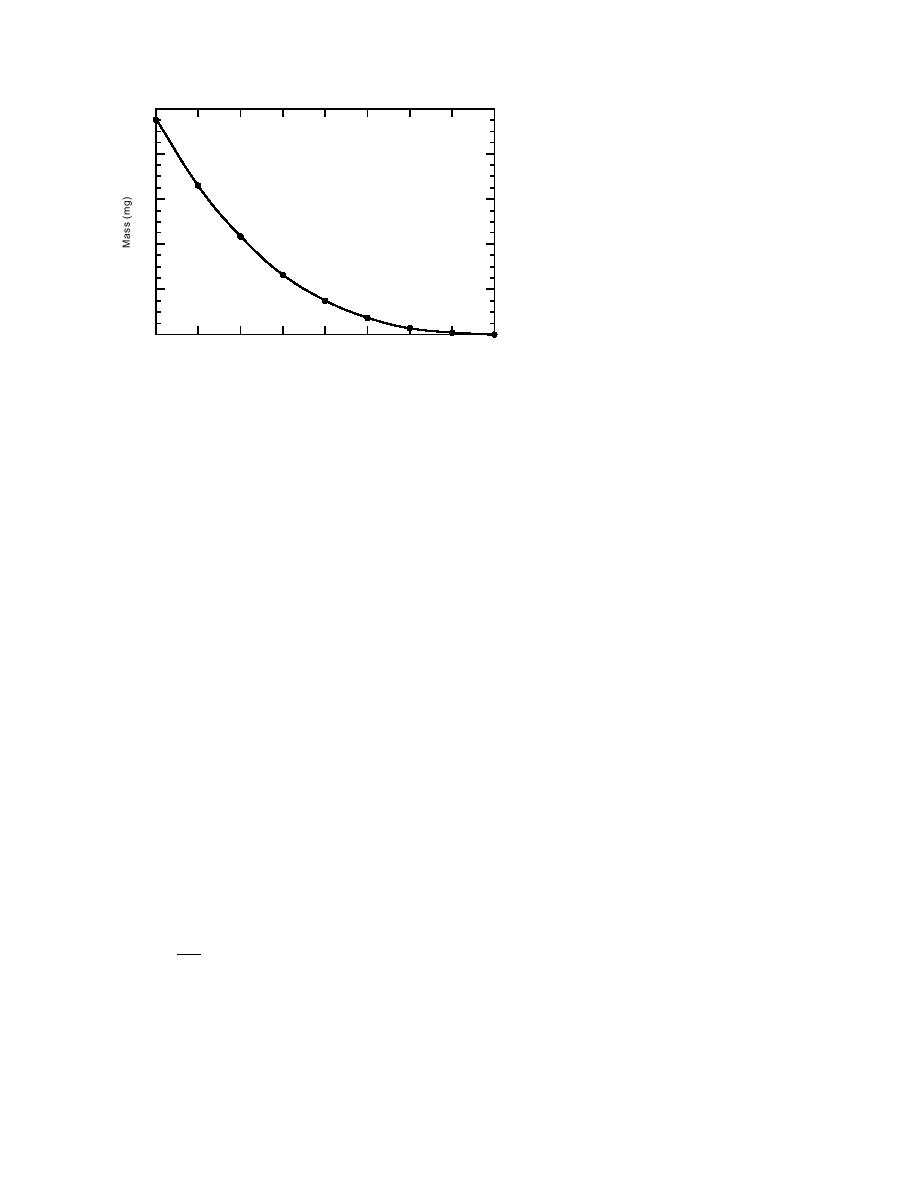
1.0
0.8
0.6
0.4
Figure 5. Calculated dissolution of a 1-mm-
diameter P4 particle in 25C water assum-
0.2
ing the rate of dissolution per unit surface
area is constant [1.2 g/(cm2 hr)]. The rate is
based on the data from Spanggord et al. (1985)
0
2
4
6
8
for the slowest flow rate tested.
Time (years)
quently held above its melting point so that it
Octanolwater partition coefficient
may be poured and molded. Because the melting
The octanolwater partition coefficient (Kow) is
point exceeds the autoignition temperature, large
the ratio of the concentration of a solute in the
quantities of water are used commercially either
organic phase (octanol) to the concentration of a
for storage and handling of P4 or for washing
solute in water at equilibrium and is often used as
equipment that has been in contact with P4. This
an indicator of the bioaccumulation potential of an
"phossy water" contains suspended (colloidal)
organic substance in fatty tissue of organisms and
and dissolved P4 (Dacre and Rosenblatt 1974).
of mobility and sorption in the environment. High
Kow values (>1000) mean a tendency for bioaccu-
Larger particles may also be suspended (Peer
1972). Release of phossy water has caused fish
mulation, low mobility and sorption to organic
kills in Canada (Idler 1969) and the United States
matter. Spanggord et al. (1985) measured Kow
(Dacre and Rosenblatt 1974).
using the method of Leo et al. (1971), where white
The aqueous solubility of P4, as measured by
phosphorus concentrations were measured in both
Stich (1953), is 3 mg/L at 15C, and this is the val-
the water and octanol phases. The mean of seven
determinations was 1200 100, indicating that P4 is
ue reported in the Handbook of Chemistry and Phys-
ics. However, colloidal suspensions have concen-
lipophilic and has the potential to bioaccumulate.
trations up to 100 mg/L (Sullivan et al. 1979).
Following the release of wastewater from a
Spanggord et al. (1985) measured the aqueous
white phosphorus production facility in New-
solubility of P4 by placing excess P4 in deoxygen-
foundland, numerous studies were performed on
ated water, heating to 45C, sonicating for 30 min-
the bioaccumulation of P4 in marine animals
utes, then stirring for 48 hours. Solutions were
(Fletcher 1971, 1973, 1974). In animals exposed to
then centrifuged at 7000 rpm for 60 minutes at
aqueous solutions of P4, white phosphorus was
three temperatures. Measured P4 concentrations
found to concentrate in fatty tissues. However, if
were 2.4, 4.1 and 5.0 mg/L at 15, 25 and 35C.
the animals survived the exposure and were
placed in uncontaminated water, the P4 concentra-
Because P4 is not an electrolyte, solubility in
tions dropped below detectable levels. Similarly
saline solutions will decrease as salinity increas-
Clarkson (1991) reported that white phosphorus
es, a process known as "salting-out" (James 1986).
has been detected in the tissues of people who
The effect is expressed mathematically by the Set-
died within a few days following ingestion of
schenow equation:
white phosphorus but not in those that die after
o
longer periods. These results are similar to those
Ms
= Ks Msalt
(7)
log
Ms
at Eagle River Flats. In carcasses of ducks from
o
where Ms is the solubility of the nonelectrolyte in
Eagle River Flats, P4 concentrations were highest
pure water and Ms is the solubility of the nonelec-
in the fat and skin (Racine et al. 1992a,b,c, 1993,
trolyte in the saline solution with a salt concentra-
Roebuck et al. 1994). Predators exposed to P4-con-
tion of Msalt. Ks is a constant, known as the Set-
taminated meat initially accumulated P4 in fatty
schenow or salting-out constant (James 1986). A
tissues; when contaminated meat was replaced by
value for Ks has not been published.
uncontaminated meat, P4 concentrations in tissues
6




 Previous Page
Previous Page
