The lower limit is not a function of temperature,
kcal) and the concentrations of P4, O2 and M. The
while the upper limit decreases with increasing
reaction between P4 and O2 is rapid when the
temperature. These observations led to the theory
denominator of the term in the parentheses ap-
of branching-chain reactions. The reaction mech-
proaches zero. Dainton and Kimberley (1950)
anism proposed by Dainton and Kimberley
stated that within the limits of the glow reaction
(1950) is:
the "rate appears only to be limited by the sup-
ply and interdiffusion of the reactants."
(1) P4 + O2 → P4O + O
initiation
Solid and liquid P4 may ignite spontaneously
(2) P4 + O + M → P4O + M
in air. The autoignition temperature at atmo-
propagation
spheric pressure for solid or supercooled liquid
(3) P4On + O2 → P4On+1 + O
branching
P4 in dry air is 38C (Dainton and Bevington
(n = 1, 2, ...9)
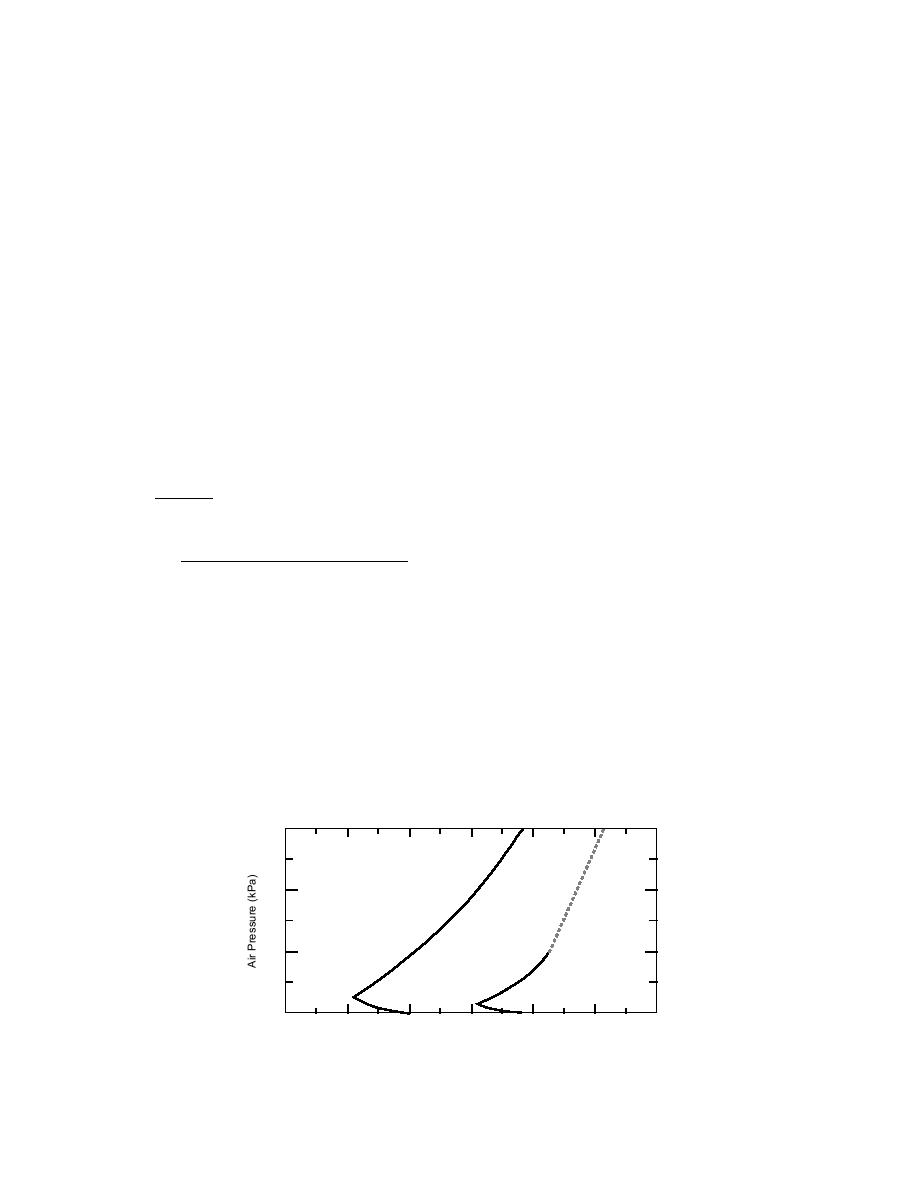
1946), and for a range of temperatures below
38C (Fig. 2), P4 will slowly oxidize, producing a
(4) O + O2 + M → O3 + M
termination
greenish glow. Below 5C, there is no reaction,
(5) O + X → stable product
termination
unless the pressure is reduced to less than atmo-
(6) O + wall → adsorbed oxygen termination
spheric.
In 1681, Slare (Mellor 1928) made the follow-
where M is a third body and X is an inhibitor. The
ing seemingly paradoxical observation:
rate equation given by Dainton and Kimberley
It now being generally agreed that the fire
(1950) is:
and flame of phosphorus have their pabulum
out of the air, I was willing to try this matter in
d[P4O10 ]
= k 1[P4 ][O2 ]
vacuo. To effect this I placed a considerable
lump of phosphorus under a glass, which I fixed
dt
to an engine for exhausting the air: then present-
(1)
ly working the engine, I found it to grow lighter
10k 2 [P4 ][M]
(i.e., the phosphorus to become more lumi-
1+
k 4 [O2 ][M] + k5 [X] + k 6- 8k 2 [P4 ][M]
nous), though a charcoal that was well kindled
would be quite extinguished at the first exhaus-
tion; and upon the third or fourth draught,
where k1,2...6 are reaction constants for the six
which very well exhausted the glass, it much
equations for the reaction mechanism. The lower
increased in light, and continued to shine with
limit of the glow reaction is due to "wall termina-
its increased light for a long time; on re-admit-
tion," reaction (6). The constant for reaction (6) is
ting the air, it returned again to its former dull-
a function of the diffusivity of oxygen atoms in
ness.
the gas mixture, the diameter of the reaction ves-
White phosphorus does not glow in oxygen at
sel and the concentrations of P4, O2 and M. The
1.5 atmospheres unless the temperature is raised
upper limit is due to homogeneous termination,
above the melting point (Mellor 1928). Several
reactions (4) and (5). The constant for reaction (4)
researchers gathered evidence that the oxidation
is a function of a frequency factor for third-body
of P4 occurs in the vapor phase. If a stream of air
reactions, temperature, activation energy (4.3
is blown over the oxidizing P4, the glow appears
150
100
Slow Reaction
Glow Region
Flame Region
50
0
60
40
20
0
20
40
60
Temperature (C)
Figure 2. Effect of temperature and air pressure on the oxidation of
solid P4. (After Dainton and Bevington 1946).
3




 Previous Page
Previous Page
