25
20
15
10
5
3
2
30
NaCI Molality (mol kg1)
20
1
10
0
10
Temperature (C)
20
0
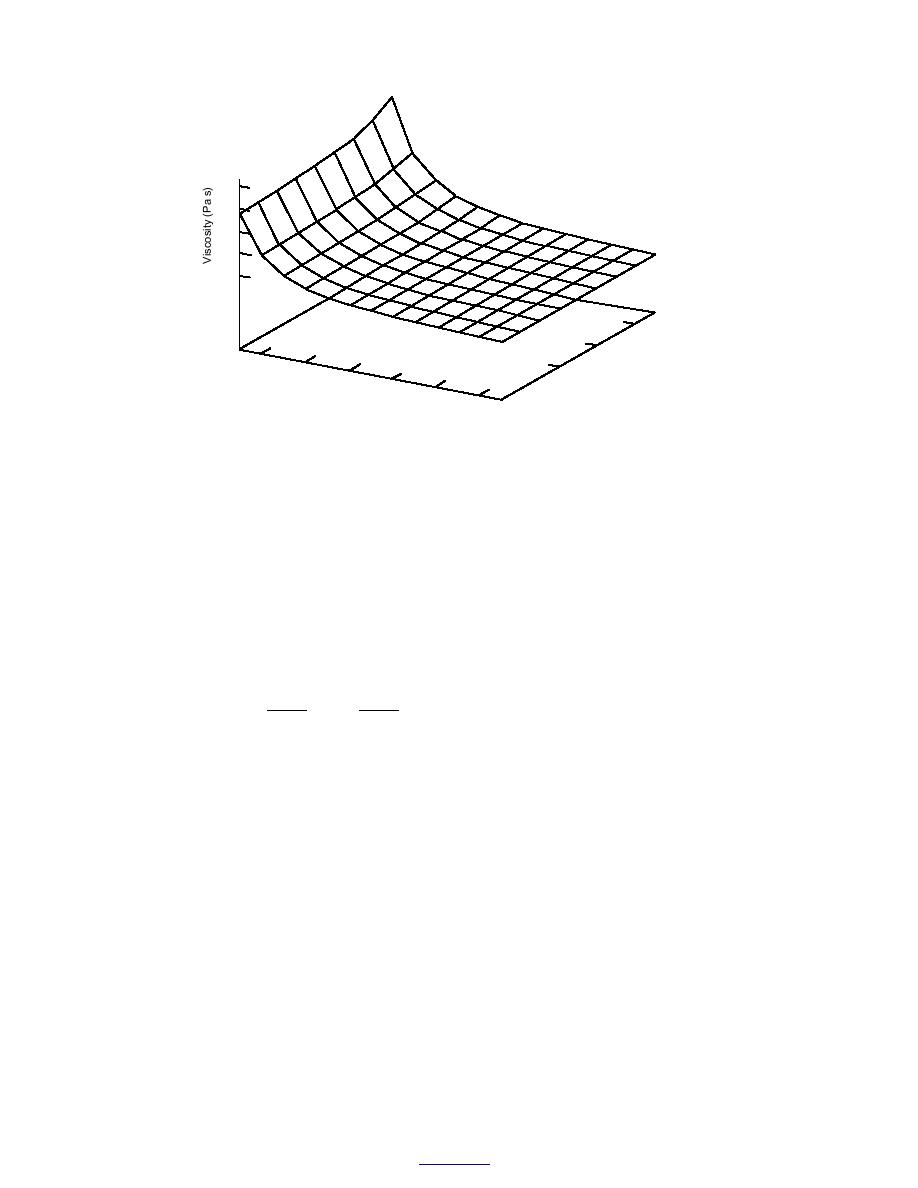
Figure 5. Predicted viscosity of aqueous NaCl solutions as affected by temperature and
solution concentration.
This relation and an equation that describes the viscosity of supercooled water (e.g., eq 6
or eq 3) allow one to predict the viscosity of electrolyte solutions at low temperatures,
though predictions at high molalites or low temperatures may be subject to significant
extrapolation errors. To the author's knowledge, there are no measurements of viscosities
of electrolyte solutions at subzero temperatures in the published literature. A plot of the
likely interaction between temperature and electrolyte molality on the viscosity of aqueous
solutions is presented in Figure 5.
Interfacial tension of liquid water against its vapor
The interfacial tension of water against air (in N m1) from the triple point of water
(0.01C) to the critical point of water (374.15C ) has been fitted to
u
T -T
T1 - T
γ wa = γ 0 1
1 - v
(9)
T1
T1
in which the parameters have the following recommended values: γ0 = 0.2358 N m1, T1 =
647.15 K, u = 1.256, and v = 0.625. While there have been few reported measurements of γwa
below 0C, these data indicate that eq 9 is approximately valid to at least 8C (Haar et al.
1984). The complexity of eq 9 belies the fact that the interfacial tension of water against its
vapor is virtually linear from 10C to 50C, as can be seen in Figure 6.
Interfacial tension of liquid water against ice
Apparently, the most precise measurement of the interfacial tension of liquid water
against ice is that of Ketcham and Hobbs (1966), who reported a value of γiw (p = 0.101325
by Jellinek (1972).
Heat capacity
Heat capacity is the fundamental physicalchemical quantity by which changes in en-
tropy and enthalpy with temperature can be calculated. Given the chemical and biological
importance of water, it is understandable that equilibrium heat capacities of pure liquid
water and ice phases have been measured precisely for most temperatures and pressures.
In recent years, the heat capacities of supercooled water have been measured with ever-
increasing precision.
8
TO CONTENTS




 Previous Page
Previous Page
