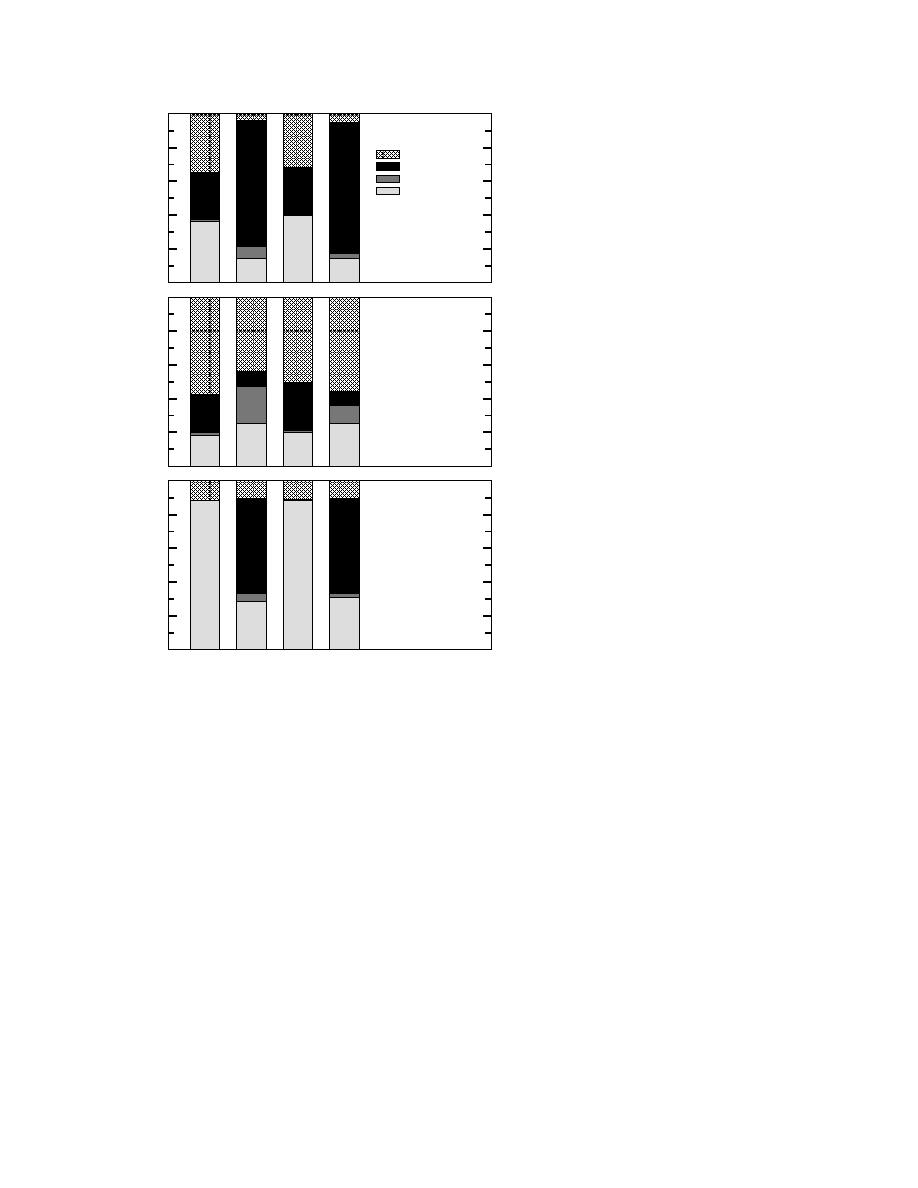
100
a consequence, the movement of explosives into
temporarily unfrozen soil is an important con-
a. RDX Mass Balance
80
sideration. It was clear from our experiments that
Fluid
Unfrozen Soil
this mobility into the temporarily unfrozen soil
Frozen Soil
60
fell in approximately the same order as the solu-
Missing
bility of explosives where picric acid >> RDX ≈
40
TNT (Fig. 4, 5). For highly soluble explosives
such as picric acid, a thicker frozen barrier may
20
be necessary to ensure containment.
0
Sequential extractions
100
In earlier work, Boitnott et al. (1997) exam-
b. PA Mass Balance
ined the effectiveness of a frozen barrier to con-
80
tain heavy-metal-laden soil during remediation by
60
sequential extraction with ethylene diamine tetra-
(%)
acetic acid (edetic acid, EDTA). They found that
40
over 90% of the Cu and Zn and over 80% of the
Cd and Ni was removed during sequential extrac-
20
tions.
In our work with explosives, 45% of the RDX
0
was recovered from the field-contaminated soil
100
(Chambers 2 and 4) with aqueous extractions
c. TNT Mass Balance
(Fig. 5a); 4456% of the picric acid was recov-
80
ered (Fig. 5b); and 11% of the TNT was recov-
ered (Fig. 5c). As a technique for cleaning an
60
explosives-contaminated soil, aqueous extrac-
40
tions were effective in removing only the highly
soluble picric acid. Other extractants such as ace-
20
tone or acetonitrile would probably be more
effective in extracting insoluble explosives such
0
as RDX and TNT. Mobility of explosives in soils
Chamber Chamber Chamber Chamber
1
2
3
4
is highly sensitive to the extractant (Selim and
Figure 5. Distribution of added explosives at the experiments' Iskandar 1994).
The greater effectiveness of the EDTA heavy-
conclusion.
metal extractions versus the aqueous-explosives
extractions was probably due to differences in solubil-
should exclude explosives from the ice phase. Taylor
ity and mobility. The concentration of heavy metals was
(1989) has estimated partition coefficients (ci/cw) for
RDX (6.06 10
3) and TNT (1.07 103) that indicate
always highest at the ice interface. This indicates that
the heavy-metal complexes were free to move toward
that ice efficiently excludes RDX and TNT. Slow freez-
the ice interface where chemical potentials should be
ing of soil to concentrate explosives such as TNT and
lowest and thereby serve as a sink for heavy metals
RDX in the unfrozen "brine" has been attempted (Ayor-
(Marion 1995). The distribution of explosives, on the
inde et al. 1989). In that trial, there was insignificant
other hand, showed no concentration increase at the ice
movement (<10%) of TNT and RDX.
interface. Instead concentrations were highest in the
There was significant movement of explosives into
upper soil layers (Fig. 4), suggesting that the explo-
the frozen barrier in Treatment 2 (Fig. 4b,d); however,
sives were not mobile and the aqueous extractions sim-
we believe that this occurred as the result of thawing of
ply removed the explosives from the lower unfrozen
the frozen soil layers that occurred when the unfrozen
layers where the sampling outlet was located (Fig. 1).
soil material was added (Fig. 2). Insignificant amounts
of explosives leached into the frozen barrier in Treat-
Stability of explosives
ment 1, where the explosives were added in aqueous
Grant et al. (1995) found that added nitramines (e.g.,
solution onto a stable frozen soil (Fig. 4a,c).
RDX) were stable over an eight-week period at all stor-
In the "real world," problems can occur in main-
age temperatures; but, added nitroaromatics (e.g., TNT)
taining a stable frozen barrier because of fluctuations
degraded rapidly at room temperature and more slowly
in electrical power or ambient temperature (Fig. 2). As
7




 Previous Page
Previous Page
