Table 4. Retention times* for explosives-related
headspace. When analysis indicated significant
compounds by RP-HPLC.
analyte in the headspace vapor, the top 5 mm of
soil in the B replicate samples was removed as
Retention time
Retention time
above. After 173 days, the final remaining soils
Analyte
(min)
Analyte
(min)
(-12C) were sampled.
1,3,5-TNB
2.51
3,5-DNT
11.11
To conduct the soil sampling, we opened the
1,4-DNB
3.88
2,6-DNT
13.61
vials and used a small scoop to carefully remove
1,3-DNB
4.93
3,4-DNT
13.86
the top 5 mm of soil. The mass of soil removed
2,4,6-TNT
5.66
2,3,6-TNT
14.13
1,2-DNB
7.07
2-ADNT
14.56
varied from about 2 to 4 g. The soil was trans-
3,5-DNA
7.84
3,4,5-TNT
16.56
ferred to a 20-mL scintillation vial and a 10.0-mL
2,4,5-TNT
8.75
4-ADNT
16.87
aliquot of acetonitrile added. The vials were
2,5-DNT
9.89
2,3-DNT
18.75
placed in a room-temperature, controlled, ultra-
2,3,5-TNT
10.80
2,3,4-TNT
40.54
sonic bath and extracted for 18 hours as described
2,4-DNT
11.05
in SW 846 Method 8330 (EPA 1995).
*Analysis conducted on a 15-cm 0.39-cm, 4-m film, LC-8
column (Waters Nova-Pak) maintained at 28C and eluted
Analyte concentrations in the soil extracts were
with 15/85 (V/V ) isopropanol/water at a flow rate of 1.4-
determined by reversed-phase high-performance
mL/min.
liquid chromatography (RP-HPLC). A modular
system was employed, consisting of a Dynatech
LC-241 autosampler with a 100-L injection loop,
times for the various explosives-related analytes
a Spectra Physics SP8810 isocratic pump, a Spec-
are presented in Table 4. Additional confirmation
tra Physics SP100 variable-wavelength UV-detec-
of analyte identities was obtained by analyzing
several of the 63-day replicate A sample extracts
tor set at 254 nm, and a Hewlett Packard 3396
on a 25-cm 4.6-mm (5-m) LC-18 (Supelco) col-
series II digital integrator. Separations were
obtained on a 15- 0.39-cm, 4-m film, LC-8 col-
umn eluted with 50:50 methanol/water at 1.5
umn (Waters Nova-Pak) maintained at 28C and
mL/min.
eluted with 15/85 (V/V) isopropanol/water at a
flow rate of 1.4-mL/min. The detector response
Vapor sampling using SPME and
vapor analysis using GC-ECD
was obtained from the digital integrator operat-
ing in the peak height mode. For both RP-HPLC
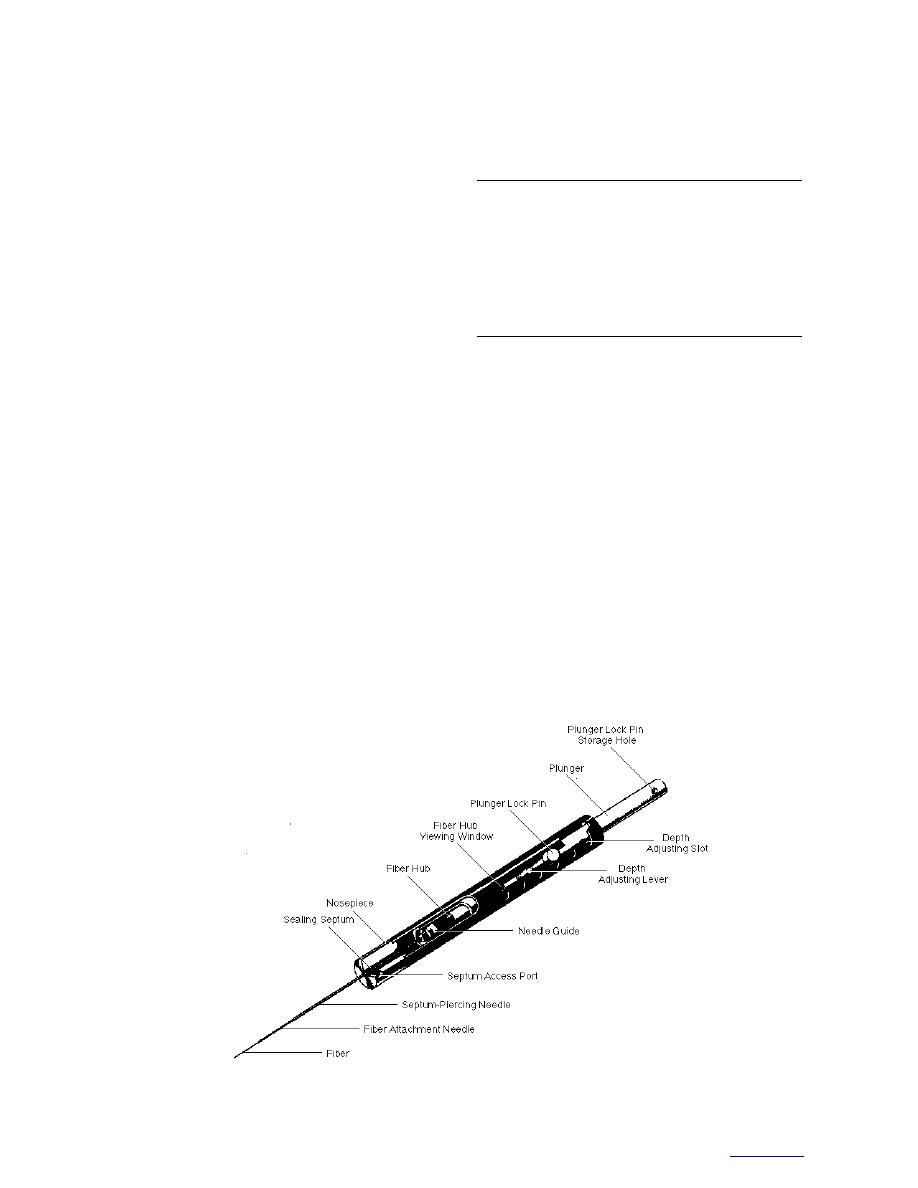
The headspace vapor was sampled by solid
phase microextraction (SPME) using an 85-m
analysis and gas chromatography with a micro-
electron capture detector (GC-ECD), standard
film coating of polyacrylate on a fused silica fiber
analytical reference materials (SARMs) were
(Supelco) (Fig. 2). We conditioned the fiber before
used in making the standard solutions in HPLC
use following the manufacturer's recommenda-
grade acetonitrile (Sigma-Aldrich). Retention
tions. Also, at the beginning of each sampling
Figure 2. Portable solid-phase microextraction (SPME) field sampler (Supelco).
4
To Contents




 Previous Page
Previous Page
