APPENDIX A: UPTAKE OF 15N-LABELED TNT TRANSFORMATION PRODUCTS
Incubation of kenaf
One kenaf plant was removed from a control pot and the soil completely washed from the roots. The
root mass was teased apart into four sections; each section was placed in a 500-mL beaker. Each beaker
was filled with water containing one of the 15N-labeled amino and diamino TNT transformation prod-
ucts. Water was replenished every day for one week. The roots were then cut from the plant, washed with
water, extracted overnight with acetonitrile, finely cut up, and air-dried.
NMR spectrometry
mm ceramic probe (zirconium pencil rotors). Chemical shifts were referenced to glycine, taken as 32.6
ppm. Acquisition parameters included a 30,000-Hz spectral window, 17.051-ms acquisition time, and
5000-Hz spinning rate. Contact times and pulse delays were 2.0 ms and 1.0 s for the pure 2,4DANT, 5 ms
and 0.5 s for the 2,4DANT-treated root, and 2.0 ms and 0.5 s for the unlabeled blank root sample. The line
broadenings (LB) in hertz are shown in the figures.
Results
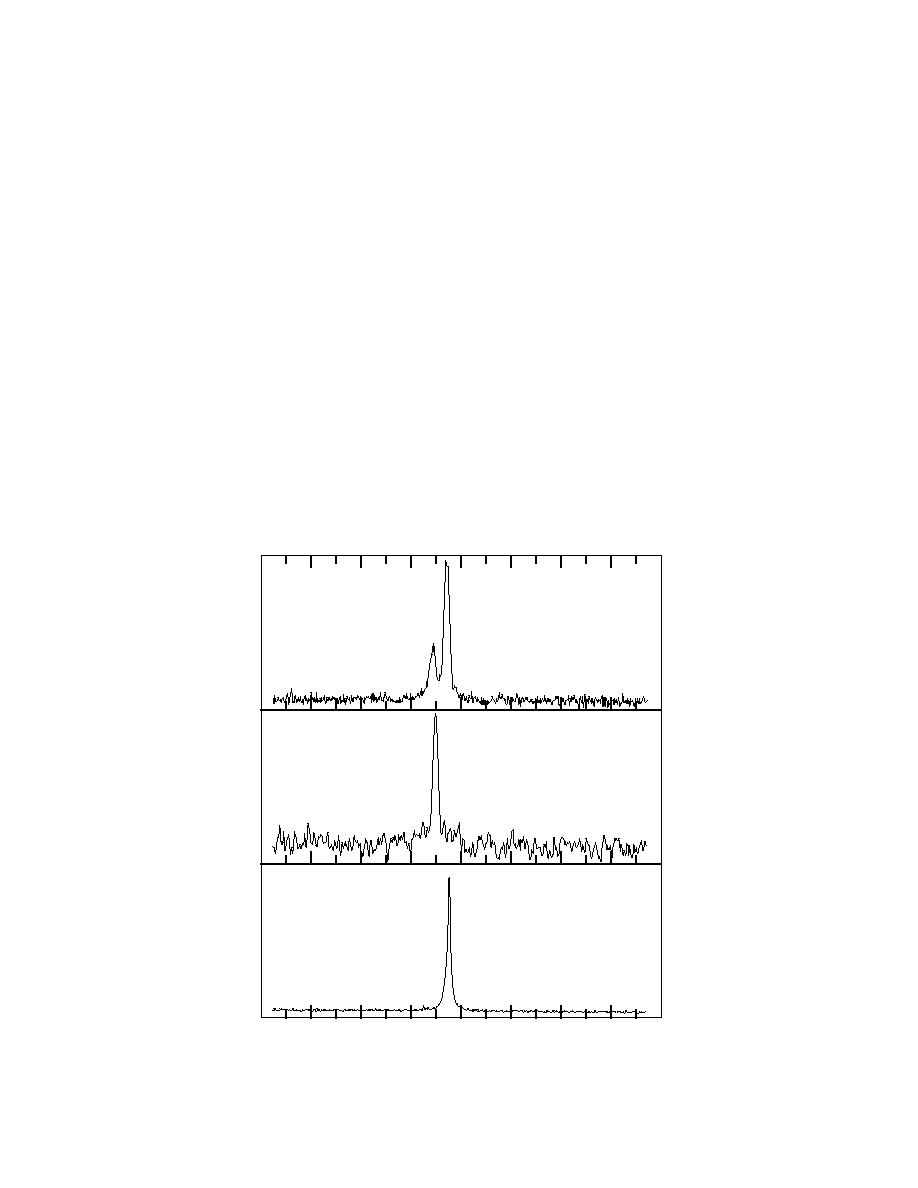
Solid state CP/MAS 15N NMR spectra were recorded on the root sample from kenaf treated with 15N-
labeled 2,4DANT, blank root, and pure 15N-labeled 2,4DANT (Fig. A1). The chemical shift position of
71.0 ppm
Kenaf Root
2,4DANT
LB = 1 Hz
117.4 ppm
117.9 ppm
Kenaf Root
Blank
LB = 100 Hz
2,4DANT Standard
57.7 ppm
LB = 1 Hz
800
600
400
200
0
200
400
600
800
ppm
Figure A1. 15N-NMR spectra of kenaf root.
9




 Previous Page
Previous Page
