describe the transport of gaseous TCE, Conant et
EXPERIMENTAL
al. (1996) used linear partitioning to characterize
Apparatus and materials
the interactions with the bulk soil matrix. This
The chambers used for these studies consisted
model considered only solidaqueous phase parti-
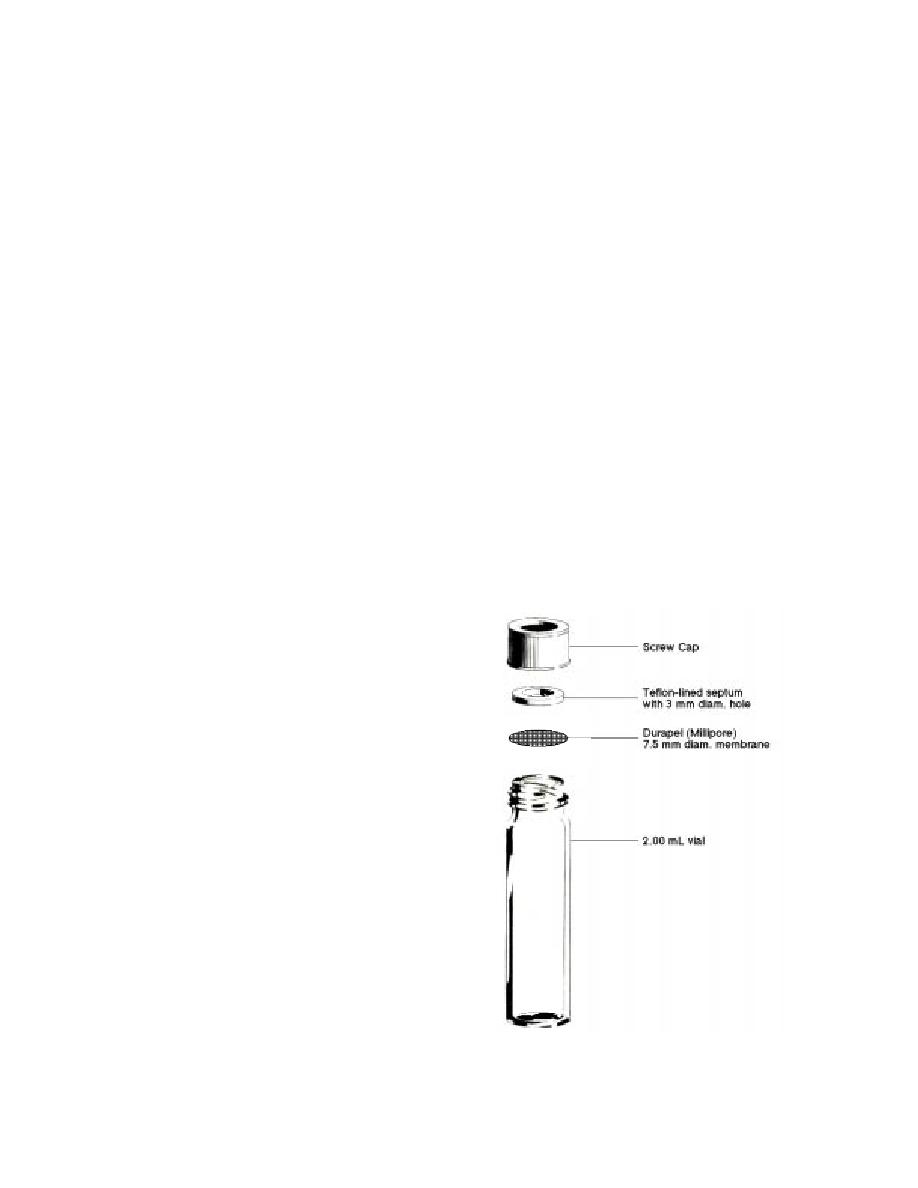
of 500-mL glass jars capped with a Teflon-backed
tioning, omitting solidvapor phase partitioning.
silicone septum cap (I-Chem), which allows for
Implicit to the omission of solidvapor partition-
syringe-needle penetration. The vessels used for
ing is that all mineral surfaces were considered to
discrete soil and liquid samples were clear glass
be hydrated, a condition that prevails in humid
2.00-mL autosampler vials with open top screw
and temperate climates. Hydrated mineral sur-
caps and PTFE-faced silicone septa (Supeloc, Inc).
faces usually exist when the bulk soil has a mois-
These small vials were modified to allow for the
ture content of a percent or more. Therefore, the
exchange of VOC vapors between the discrete
model assumes that the distribution of VOCs be-
sample and the chamber atmosphere, while limit-
tween the vapor phase and bulk soil can be
ing the transfer of water vapor. Gaskets were
described by a proportionality constant, analo-
made out of the septa by punching a 3-mm-diam.
gous to the Henry's law constant, i.e., the ratio be-
holes out of the center of each. Hydrophobic
tween vapor and aqueous VOC concentrations
membranes 7.5 mm in diameter were then
under equilibrium conditions. With regard to the
punched out of a 20- 20-cm sheet (≈ 4-mil) of
sorption capacity of the bulk soil matrix for VOCs,
Durapel (Millipore). When in use, these hydro-
Conant et al.'s model considered the organic car-
phobic membranes were placed below the septa
bon content to be the dominant variable, with soil
(PTFE face adjacent to membrane). In this arrange-
moisture content playing a smaller role.
ment the Durapel membrane disk is pressed be-
This study describes a laboratory approach for
tween the rim of the glass vial and the Teflon-
assessing vapor-water and vapor-soil partition-
faced septum gasket (Fig. 1).
ing of VOCs under conditions typical of the sub-
The chamber also contained two 20-mL glass
surface in temperate climates. Notable differences
bottles, one of which contained a vapor fortifica-
from most previous studies are the use of expo-
tion solution and the other contained 10 mL of
sure periods of three or more weeks, quiescent
conditions, hydrated mineral surfaces, and a con-
stant temperature of 11 1C. The intent of these
experiments was not to assess the transport
characteristics of VOCs, but to estimate the quasi-
equilibrium concentration relationships that are
likely to exist among vapor, water, and soil grab
samples.
OBJECTIVE
The objective of this study is to better under-
stand the concentrations of VOCs that exist among
the vapor, water, and bulk soil media. To achieve
this goal, VOCs were passively transferred by a
vapor fortification process (Hewitt and Grant
1995) to these three different media, held at
11 1C in a vapor-tight chamber. Furthermore,
relatively long exposure periods (>21 days) were
used in an attempt to create a quasi-equilibrium
condition for vapor-bulk soil partitioning. The
equilibria are only considered to be quasi, because
VOC sorption has been assumed to follows a simi-
lar biphasic process as desorption (Steinberg et al.
1987). Therefore, soils most likely continue to sorb
VOCs over a very long time (on the order of
months to years) because of diffusion-limited pro-
Figure 1. Modified vial used for holding soil and
cesses occurring within the soil matrix.
water samples in the exposure chamber.
2




 Previous Page
Previous Page
