1.0
a study that tested types 304 and 316 SS cas-
ings showed that these casings were slight-
ly sorptive (e.g., 10% loss in 24 hours) under
0.8
similar conditions (Parker et al. 1990).
0.6
Because Cr was added to the groundwa-
ter as dichromate, a negatively charged spe-
0.4
cies, we would expect the results for this
PVC
anion to be similar to those for As. Statisti-
PTFE
FEP
0.2
cal analyses of these data indicated that loss-
FRE
es due to sorption were not significant for
FRP
any of the casing materials, except for a few
0
16
32
48
64
80
of the samples exposed to the FRE casings
Contact Time (hr)
(Table A2). However, these losses were slight:
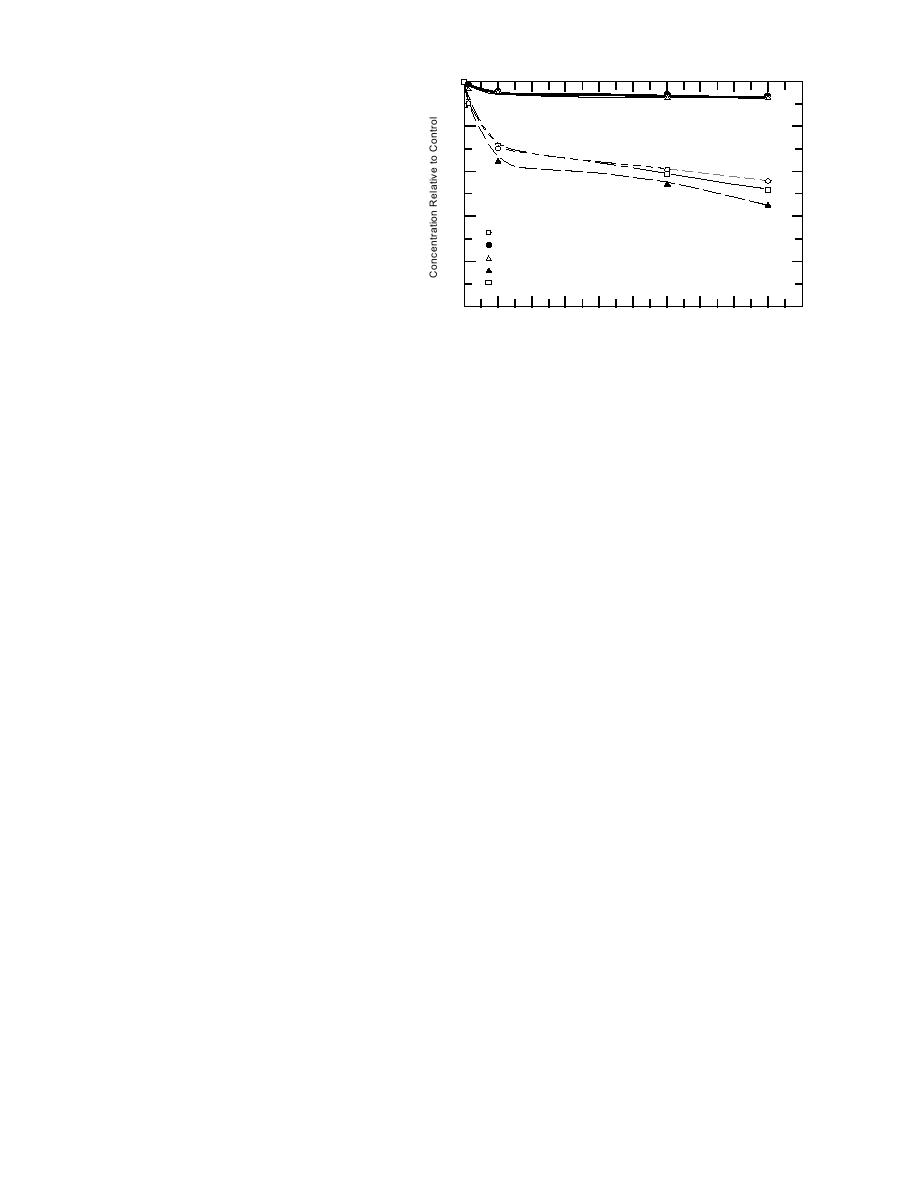
Figure 1. Sorption of lead by well casings.
less than 5% (Table 5).
In similar studies that examined sorption
of Cr by SS 304 and 316 casings, there was some
shown that this analyte is readily sorbed by SS,
slow sorption by SS 316 casings (e.g., 13% in eight
PVC, and PTFE well casings. Table 5 and Figure 1
hours) (Parker et al. 1990).
show that there was substantial sorption of Pb in
the samples exposed to the PVC, FRE, and FRP
casings. These losses were significant after one
Cations
hour of exposure (Table A4). Sorption tended to
Cadmium
be significantly greater for FRE than FRP or PVC.
While the polymeric casings did not tend to
By the end of the study (72 hours), mean losses
sorb anions, this was not the case for cations.
for these materials were 57% for FRE, 48% for
FEP was the only polymeric material of the five
FRP, and 44% for PVC. After 24 hours, concentra-
tested that had no significant effect on Cd con-
tions of samples exposed to the fluoropolymers
centrations. PVC was the most sorptive material
(FEP and PTFE) were also significantly lower than
tested--losses ranged from 7 to 21% (Table 5), and
the controls, indicating that sorption had occurred.
these losses were significant for all four sampling
However, these losses were small (2 to 7%).
times (Table A3). Also, losses of Cd became sig-
Sorption by the most sorptive material, FRE,
nificant after eight hours for samples exposed to
appears to be equivalent to what has been ob-
FRE (9% loss) and after 24 hours for samples ex-
served under similar conditions for SS 304 and
posed to FRP (10% loss). Cd concentrations were
less than what has been observed for SS 316 (Park-
also significantly lower in samples that were ex-
er et al. 1990). Although we do not have any ex-
posed to the PTFE casings after eight and 24 hours
planation, sorption of Pb by PVC appears to be
(~5 to 7% loss), but not by the end of the study.
considerably greater in this study than what was
There was less sorption of Cd by the PTFE, FRE,
observed in a previous study (Parker et al. 1990).
and FRP casings as the study progressed. This
may be because, as the leaching study showed,
these materials leach low levels of this analyte.
CONCLUSIONS AND
In a similar study, both types of SS casings (304
RECOMMENDATIONS
and 316) leached Cd and therefore no sorption
was observed (Parker et al. 1990). However, un-
These studies show that, with respect to leach-
der low DO conditions, SS 304 was very sorptive
ing, the fiberglass materials were more apt to leach
of Cd (losses ranged from 18% at eight hours to
higher concentrations of contaminants than the
60% at 72 hours) (Hewitt 1992).
other three materials. However, in at least one
case (Ni), we suspect that this may be the result of
Lead
an experimental artifact. With the exception of Cd,
Since lead also exists as a cation in solution, we
the performance of PVC was almost as good as
would expect that some of the casing materials
the fluoropolymers. The concentrations of leached
would sorb Pb in a fashion similar to Cd. Previ-
contaminants were relatively low and did not ap-
ous studies (Parker et al. 1990, Hewitt 1992) have
proach limits set by the US EPA for drinking wa-
10




 Previous Page
Previous Page
