electrical power of ice does not exceed 4 mV/C (Bry-
controlled within 0.2C. All ice samples were ma-
ant and Fletcher 1965), this effect is always small.
chined with a cutter to an outer diameter of 10 cm and
Takahashi (1969a) measured the electrical potential
then subsequently polished with sandpaper. The ice
difference between a vibrating metal plate and the sur-
and 107 Ω-1m1 at T = 10C. The measurements of
face of pure single crystals of ice, both before and after
σs were made both with the apparatus at rest and dur-
the ice was rubbed with another single crystal or with
ing experiments. No difference was found among σs
a plane. He found changes in the potential difference
ranging up to 0.20.3 V after the rubbing. Unfortunately,
values measured before, during and after the friction
he did not realize that he simply produced an icemetal
experiments. This showed that possible local melting
contact potential or, in other words, he measured a dif-
and refreezing of the ice surface does not significantly
ference in electron work functions between the metal
change the ice conductivity. When the cylinder rotated,
a frictional force changed the belt tension (T2 T1).
plate and the ice. The observed relaxation time was
very long (hours at 10C) and had nothing in common
with the dielectric relaxation time in ice, which is 5
T - T
105 seconds at that temperature. The effect that Taka-
1
=
arcth 2 1
(1)
α
T1 + T2
hashi found is likely ascribable to a difference in the
structure and thickness of the surface charge double
where α is an angle shown in Figure 1.
layer on fresh and aged ice surfaces. The layer slowly
changed by adsorption of impurities from the air and
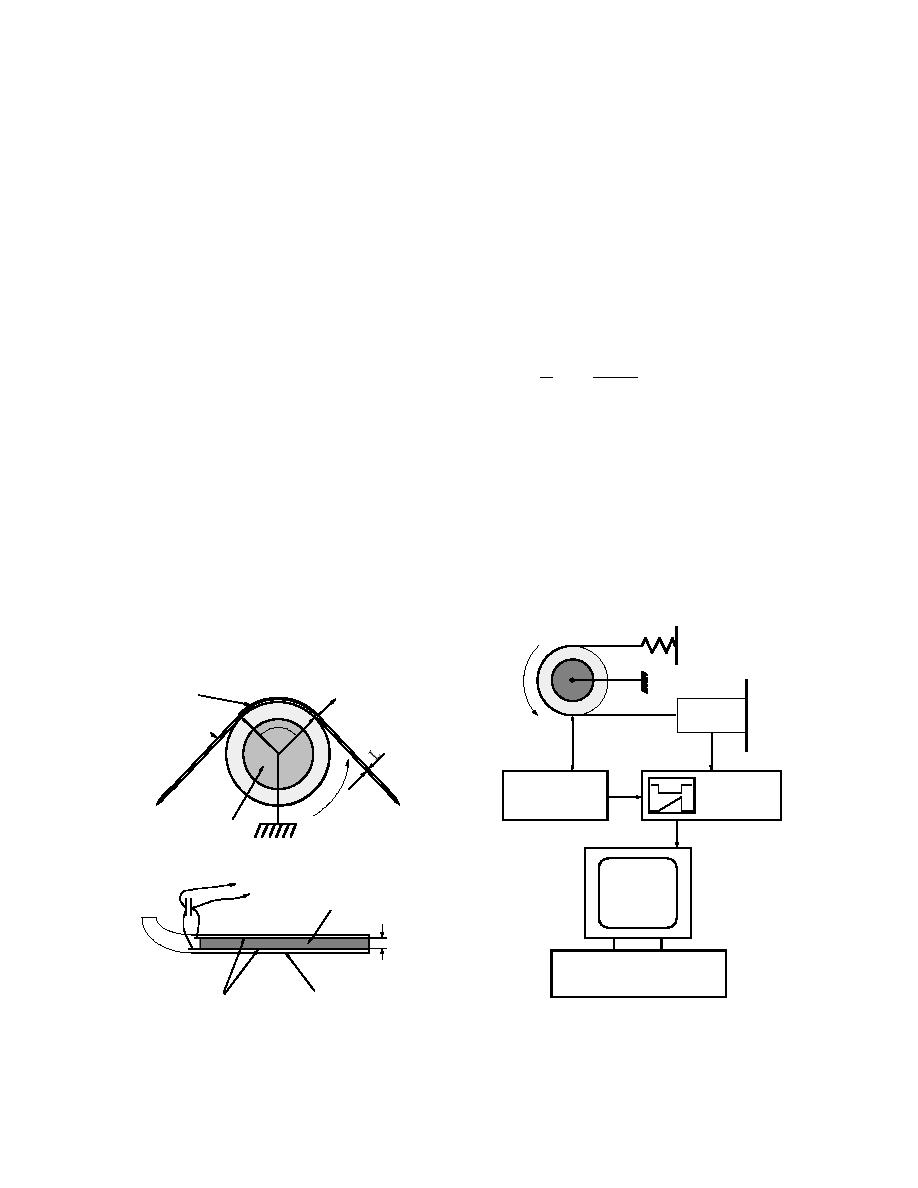
A schematic of the experimental setup is shown in
diffusion of the impurities in ice. We will discuss such
Figure 2. Reverse rotation of the cylinder allowed us to
measure T2 instead of T1. Polyethylene, aluminum and
an electric double layer in the next section.
stainless steel belts of 2.5-cm width were used as the
sliders, and measurements were made at temperatures
Frictional electrification
from 5 to 35C and at sliding velocities from 0.5 to 8
This writer (Petrenko and Colbeck 1995) studied
frictional electrification on cylindrical samples of
m/s.
polycrystalline ice grown from very pure, deionized
To determine whether friction causes melting, a
and degassed water. A polycrystalline ice layer, of
thin thermocouple (0.1 mm) was attached to the outer
about 2 cm thickness and a typical grain size of 512
mm, was frozen onto the outside of a stainless steel
spring
cylinder (Fig. 1). The cylinder was mounted on a lathe
T2
located inside a coldroom where temperature could be
to an electrometer
metal foil
T1
Ice
Load cell
dielectric film
α
Electrometer
Digital storage
ice
T1
Keithley 616
T2
stainless steel
cylinder
Electric ground
to the data logger
C(2F)
13-mm-thick dielectric plate
d
Computer
1-mm plastic plate
metal plates
Figure 2. Experimental setup (after Petrenko
Figure 1. Ice friction and ice electrification meas-
and Colbeck 1994).
urements in the laboratory (top) and on snow in
the field (bottom) (after Petrenko and Colbeck
1994).
3




 Previous Page
Previous Page
