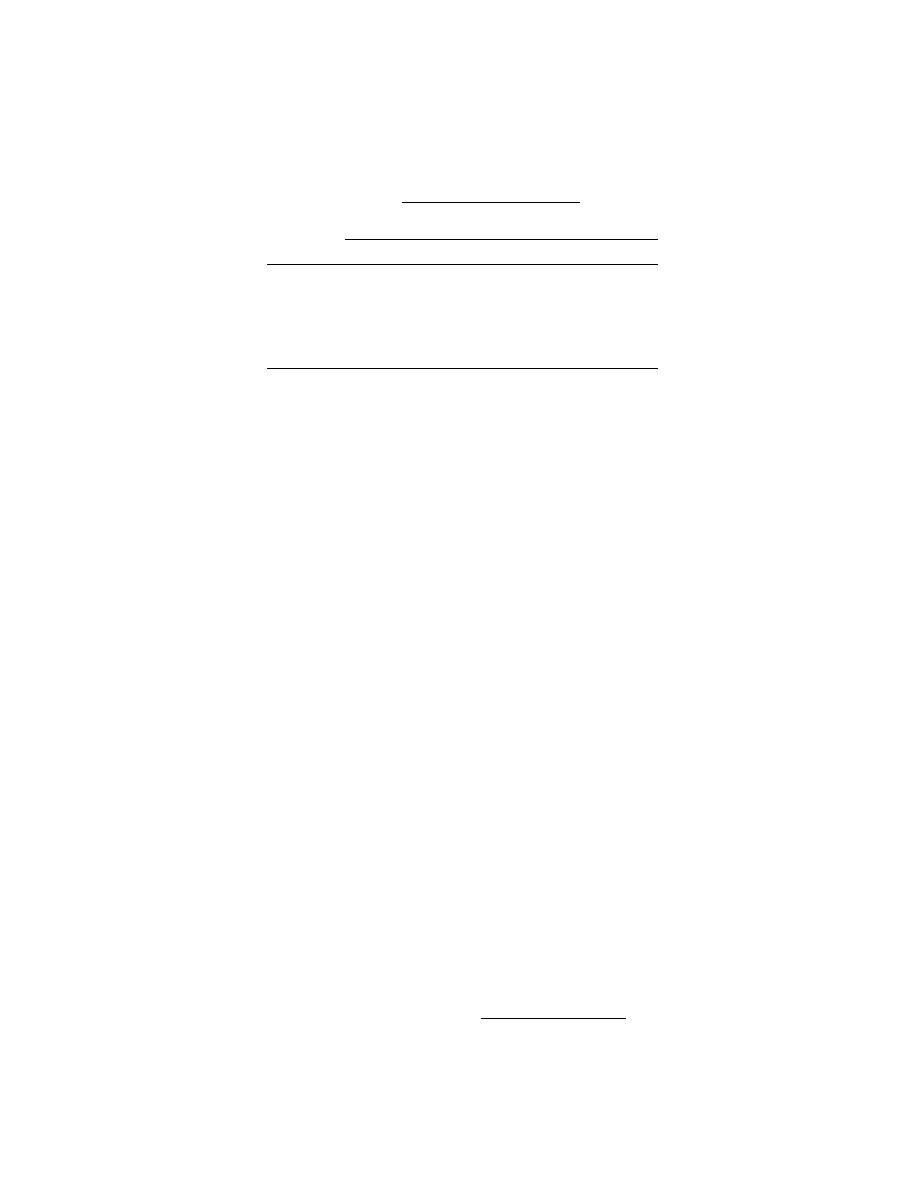
Table 7. Set 4, trial 5. Mean and standard deviations of tripli-
cate gasoline concentrations of spiking solution (g) after 1
day of refrigerated storage, and for gasoline concentrations
(g/g) in preserved and unpreserved samples stored at 22
and 4C.
Treatment aliquot
(g)
Analyte
120.1
gasoline
Storage period
Day 0†
Analyte
Day 5
Day 10
Day 14
Day 28
A. 22C --unpreserved (g/g)
110.7
6.70.5
0.60.1
0.090.01
Gasoline
ND
B. 4C--unpreserved (g/g)
110.7
110.4
8.90.5
9.51.7
7.80.9
Gasoline
C. 22C--preserved with NaHSO4 (g/g)
100.5
100.3
8.80.2
100.1
9.80.4
Gasoline
* ND = not detected, less than 0.02 g of VOC/g
† Same set used for day 0 values for unpreserved samples
Refrigeration (4C) slowed the rate of degrada-
polymerized or was chemically transformed into
tion losses, but both Ben and meta-xylene (m-Xyl)
an alcohol.* Since there was not enough water
showed substantial reductions (>80%) within a
present to create a slurry condition, NaHSO4 may
14-day storage period (Fig. 5, 8 and 12). With the
be either present as a salt or an acid. An addition-
exception of o-Xyl, all of the other aromatic hy-
al experiment not reported here showed that the
drocarbons were also substantially reduced (>95%)
loss of styrene was unique to soil samples pre-
in concentration over a 28-day period (Fig. 8, 12
served with NaHSO4; i.e., no losses were seen in
and 13). These findings and others (Hewitt 1994,
laboratory water that was similarly preserved and
Hewitt 1995a,b, Turriff 1995), suggest that refrig-
stored. Thus, the chemical reaction that transforms
styrene most likely is catalyzed by the soil. Clear-
eration is not a sufficient means of eliminating
ly, soil sample preservation by NaHSO4, or per-
microbial degradation effects on VOC analyte con-
haps any acid, would not be compatible for inves-
centrations in soil samples awaiting analysis.
tigations where styrene is a constituent of interest.
In contrast, with the exception of styrene, all of
Although these experiments used only labora-
the aromatic analytes tested and the majority of
tory-fortified samples, field samples should be-
compounds present in gasoline were preserved
have similarly because chemical preservatives in-
with NaHSO4 (Fig. 3, 6, 9, 14 and 15). The small
hibited the activity of the indigenous soil microbes.
(<30%) concentration reductions that were ob-
There are, however, some issues that need to be
served relative to day 0 can be partly attributed to
addressed aside from the chemical transforma-
slow sorption by the soil organic matter and lack
tion of styrene in soil due to preservation with
of an equilibration period between treatment and
NaHSO4:
the initial analysis. Additional evidence for this
1. How should the samples be collected?
mechanism is shown by the greater losses for com-
2. Are there any effects due to storage of sam-
pounds with the largest o/w partition coefficients
ples in VOA vials?
(n-propyl benzene [n-PB], iso-propyl benzene [iso-
3. Do all soil samples require chemical preser-
PB], 1,3 dichlorobenzene [1,3 DCB], and n-BB), and
vation?
by the trend showing that the greatest reductions
4. Is it important to obtain a pH of 2 or lower
in concentrations almost always occurred between
throughout the sample to inhibit microbiological
the first two analyses (day 0 to day 3 to 5, trials 1
degradation?
through 3). Since a equilibrium condition most
While losses of greater than 80% for some ana-
likely has already been reached for environmen-
lytes may be attributed to biodegradation when
tal samples, VOC losses of this nature would not
soil samples held refrigerated for 14 days, much
be anticipated for samples taken during a site
investigation and preserved with NaHSO4.
Styrene was not stable in the soil preserved
* Personal communication with Thomas F. Jenkins,
with NaHSO4, perhaps because it either rapidly
CRREL, 1995.
9




 Previous Page
Previous Page
