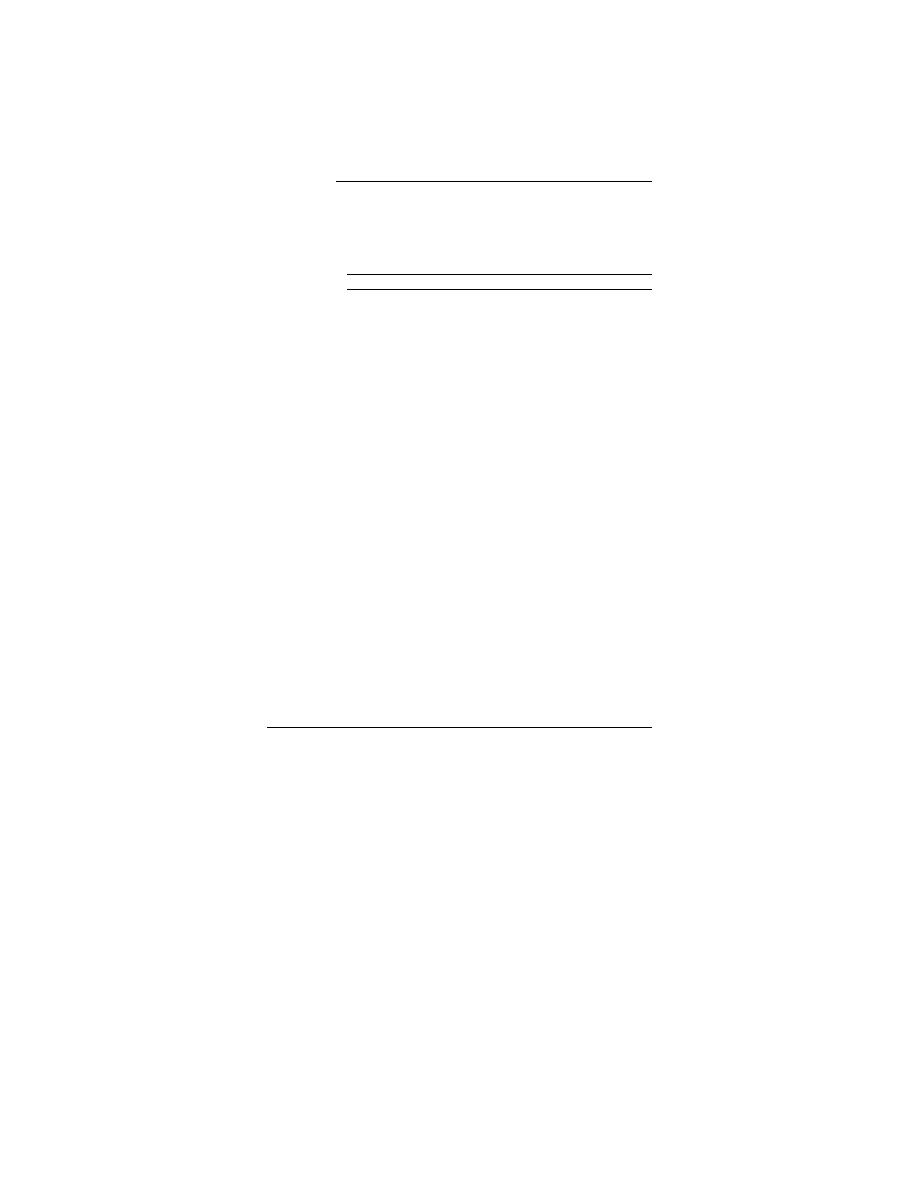
Table 4. Set 2, trial 2. Mean and standard deviations of
triplicate analyte concentrations of spiking solution (g)
and of analyte concentrations (g/g) for preserved and un-
preserved samples stored at 22 and 4C.
Treatment aliquot
Treatment aliquot
(g)
(g)
Analyte
Analyte
110.3
160.5
MC
BDCM
120.4
160.5
1,1 DCA
1,1,2 TCA
120.4
9.90.4
CF
CB
130.5
120.6
1,2 DCA
m-Xyl
Storage period
0†
Analyte
Day
Day 4
Day 8
Day 14
Day 28
A. 22C--unpreserved (g/g)
120.5
110.3
110.4
100.2
110.4
MC
120.3
120.3
120.4
110.2
120.4
1,1 DCA
120.4
120.4
120.4
110.2
110.3
CF
130.5
120.4
120.2
100.2
100.1
1,2 DCA
160.3
151.0
140.0
140.3
140.2
BDCM
160.2
140.5
130.6
130.5
140.3
1,1,2TCA
8.70.3
8.30.3
7.80.3
7.50.2
7.40.1
CB
100.5
m-Xyl
ND
ND
ND
ND
B. 4C--unpreserved (g/g)
120.5
110.2
110.2
110.5
110.2
MC
120.3
120.4
120.3
110.5
120.1
1,1 DCA
120.4
120.2
120.1
110.5
110.1
CF
130.5
120.3
120.2
120.4
120.2
1,2 DCA
160.3
140.3
150.4
140.9
120.2
BDCM
160.2
140.5
140.4
140.4
140.4
1,1,2 TCA
8.70.3
8.10.2
8.00.2
7.60.2
8.20.1
CB
100.5
9.30.3
9.10.2
0.400.50
m-Xyl
ND
C. 22C--preserved with NaHSO 4 (g/g)
120.4
120.2
120.4
130.1
130.1
MC
140.6
130.3
130.4
120.1
130.1
1,1 DCA
130.5
130.2
130.3
120.2
130.2
CF
140.3
140.1
140.4
130.3
130.1
1,2 DCA
170.2
170.4
170.9
160.5
170.5
BDCM
160.5
160.2
150.3
150.6
160.8
1,1,2 TCA
9.40.4
8.40.1
8.10.3
7.50.2
7.70.1
CB
110.4
9.90.3
9.304
8.70.3
9.30.2
m-Xyl
* ND = not detected, less than 0.02 g of VOC/g.
† Same set used for day 0 values for unpreserved samples.
could not be compensated simply by matching
that the analytes with o/w partition coefficients
of less than 2.6 had concentrations in the pre-
the solution matrix between samples and stan-
served soil samples that were consistently greater
dards (i.e., using matrix modifiers). Despite these
trends, the sample treatment precision and the
than the spiking solution. These two findings are
consistent with analyteorganic carbon partition
levels established for the analytes in the soil sam-
phenomena (Chiou 1989) and salting out (Ioffe
ples were adequate for assessing VOC concentra-
and Vitenberg 1982). Briefly, the greater the oc-
tion stability.
tanolwater partition (o/w) coefficient the great-
Consistent with previous studies where sam-
ples were held in either sealed glass ampoules or
er the tendency for an organic compound to parti-
capped VOA vials (Hewitt 1994, Hewitt 1995a,b),
tion with the organic matter present in a given
only small decreases were observed (<35%) for
soil, while salting out affects the solubility of a
the chlorinated compound concentrations. This
VOC. Since the differences in concentrations be-
trend was fairly independent of storage condition
tween the spiking solution and that of the pre-
served samples were products of both organic
or preservation, and was believed to be caused by
matter partitioning and salting out, these changes
slow soil sorption (Chiou 1989). To support this
6




 Previous Page
Previous Page
