much bigger effect on the concentration of the
Half of these replicate solutions were stored up-
VOCs than biological degradation. However, if
right and half were stored inverted. Triplicate sam-
measures are taken to limit exposure and main-
ples of each type were removed and analyzed
tain the structure of the soil during collection,
after 7, 14 and 21 days of storage, and analyte
and if the soil sample is transferred to a vapor-
concentrations were determined based on a fresh
tight bottle from which it can later be analyzed,
standard prepared from the same stock solution
then additional precautions to prevent biolog-
used to prepare the samples. In this case all analy-
ical degradation should be taken.
ses were performed on a field-portable Photo-
All the samples in this study were stored in
Vac 10S10 gas chromatograph (Hewitt et al. 1992).
sealed glass ampoules, vessels that do not lend
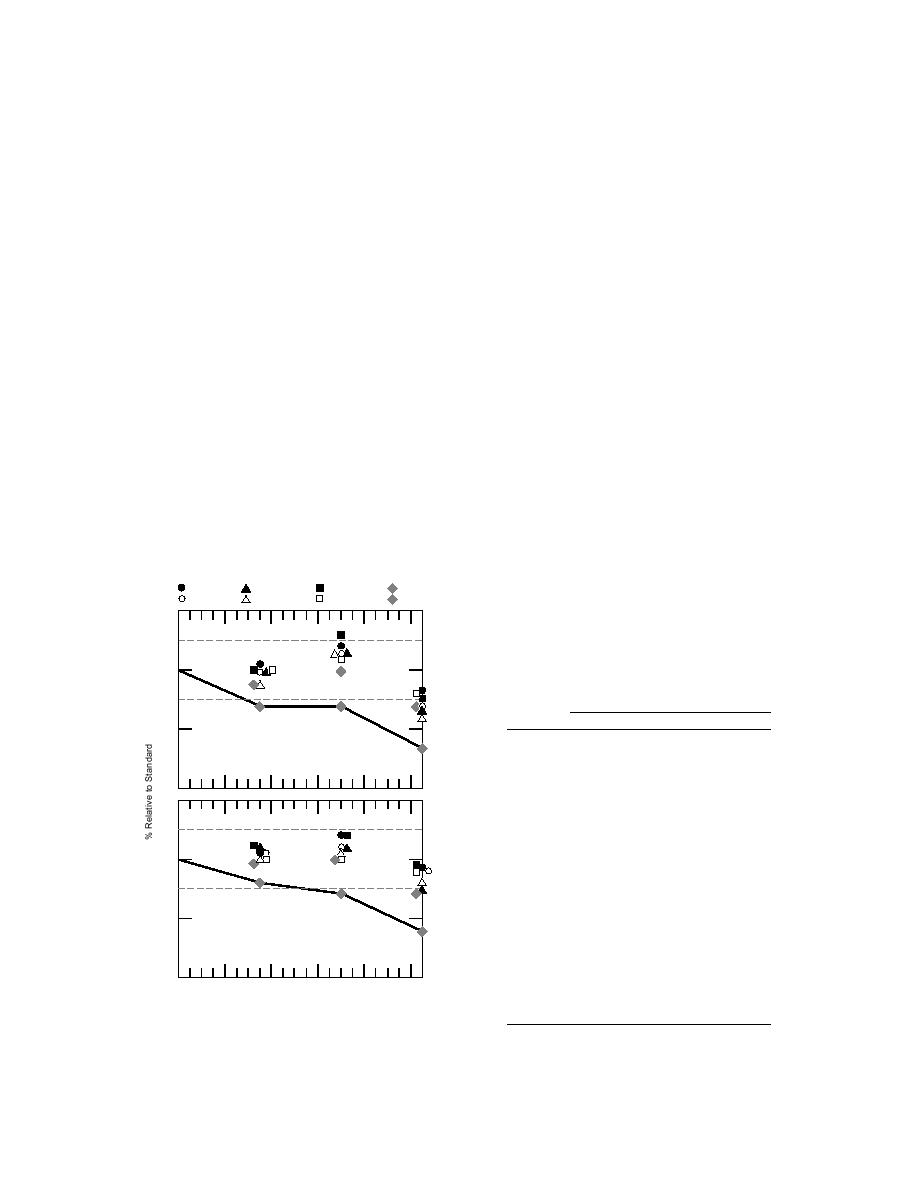
The results of this experiment showed that PCE
itself to field sampling practices, and to which
in either the gaseous or liquid phase tended to be
lost from the vials (Table 8, Fig. 16). Similar re-
samples could not be transferred without signifi-
sults were established for unpreserved aliquots of
cant volatilization losses. One of the more com-
the spiking solution transferred to 20-mL auto
mon vessels for soil sample collection, storage and
sampler VOA vials with Teflon-lined caps
shipping is VOA vials. These vials have a Teflon-
(Wheaton, Table 6). Perchloroethylene had been
faced silicone septum for the purpose of sealing
identified as one of the analytes showing the great-
and inhibiting VOC losses; however, this poly-
est rate of loss in earlier solution studies (Parker
meric material has been shown to sorb VOCs from
and Ranney 1994, Parker and Ranney in press).
solution (Gilham and O'Hannesin 1990; Parker
Based on these findings we can assume that some
and Ranney 1994; Parker and Ranney, in press).
small losses (5 to 15%) will be incurred when ei-
To see if VOCs associated with soils stored in a
VOA vial would also tend to be lost to Teflon, the
ther a soil or liquid sample contaminated with
following experiment was performed. Eighteen
VOCs is stored for an extended period (28 days)
aqueous samples of the set 1 analytes were pre-
in a VOA vial with a Teflon-lined cap. However,
pared by spiking 30 mL of laboratory water pre-
the probable loss mechanism results from the abil-
served with 0.25 g of NaHSO4 and stored refriger-
ity of VOCs to pass through this material and not
ated (4C) in 40-mL VOA vials (Eagle Picher).
because of sorption (Barbeau et al. 1995). Sorption
Ben
E-Ben
o-Xyl
TCE
Tol
p-Xyl
TDCE
PCE
110
Table 8. Response ( 100) relative to fresh
standard of solutions preserved with
NaHSO4 and held refrigerated in either
100
an upright or inverted position.
Storage period
Analyte
Day 7
Day 14
Day 21
90
Upright
99.11.0*
1024.9
96.23.6
VOA Vial, Upright
TDCE
1010.1
1045.3
96.50.0
Ben
97.70.7
1004.6
94.1 5.2
TCE
110
99.71.1
1034.8
95.21.7
Tol
94.30.6
93.94.6
86.96.1
PCE
99.412
1037.12
93.43.2
E-Ben
97.71.7
1032.3
92.05.5
p-Xyl
100
1000.4
1061.7
95.32.4
o-Xyl
Inverted
99.71.1
1000.8
97.72.7
TDCE
90
1011.4
1042.1
98.62.0
Ben
99.30.7
1002.1
94.42.4
TCE
VOA Vial, Inverted
1011.0
1022.2
97.90.9
Tol
95.90.7
94.21.4
87.73.6
PCE
1020.8
1023.1
94.83.1
E-Ben
12
16
20
0
4
8
99.90.7
1013.2
96.31.4
Holding Period (days)
p-Xyl
1021.6
1045.8
98.72.7
o-Xyl
Figure 16. Aqueous solution of set 1 analytes
preserved with NaHSO4 and stored at 4C.
Average and standard deviations n = 3.
13




 Previous Page
Previous Page
