200
tion. All of these samples were stored in 40-mL
glass vials with Teflon-lined caps and analyzed
on day 0 (the day they arrived in the laboratory)
and at 7, 14, 21, and 28 days after holding at 4C in
the dark. Samples were diluted 1:1 with methanol
160
and filtered prior to analysis. Analytical results
are presented in Appendix A.
Of the nine unfortified groundwater samples
from the NSWC, seven contained detectable con-
centrations of HMX and RDX, four had detectable
TNT, six had detectable 2ADNT/4ADNT, two had
120
a very low but detectable TNB concentration, and
one acidified sample had a very low concentra-
tion of 2,4-DNT. As observed for the fortified wa-
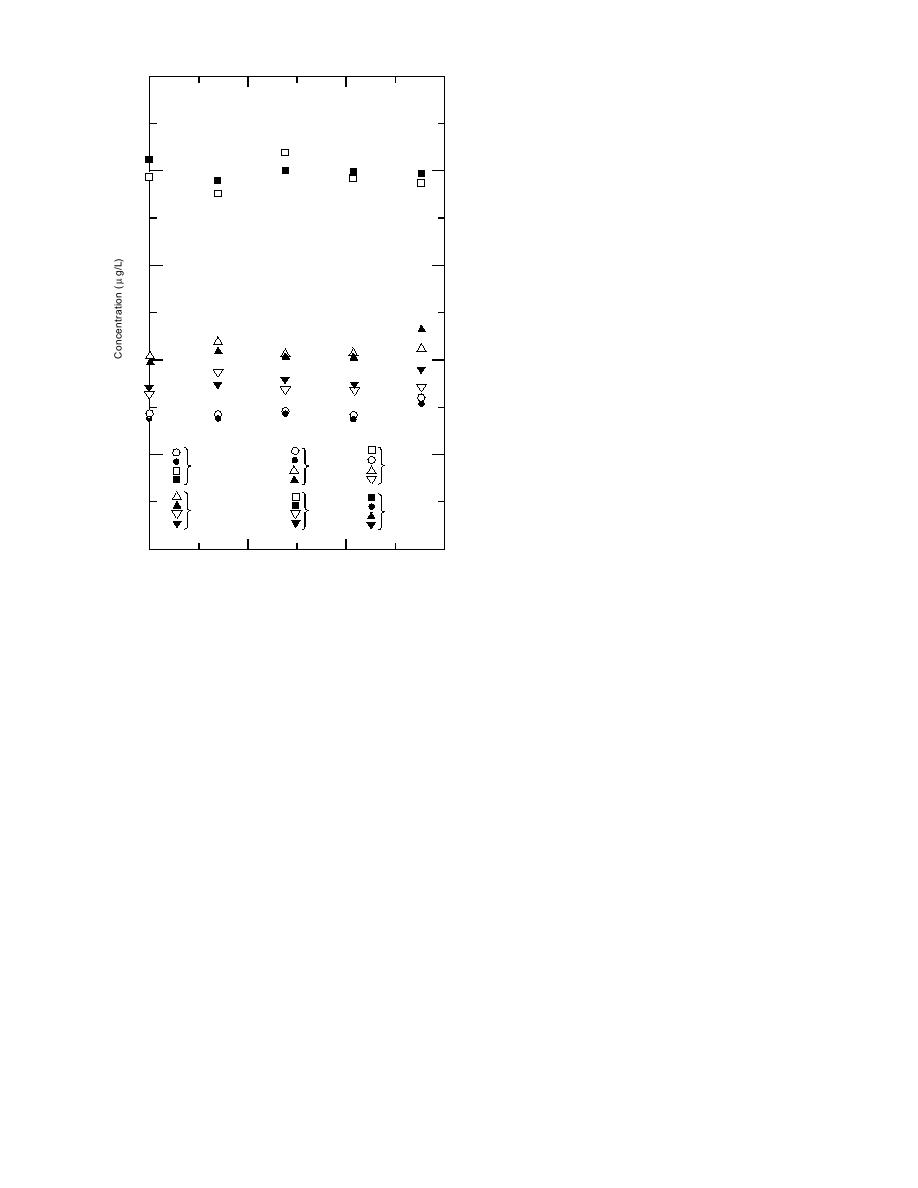
ters discussed earlier, HMX and RDX were stable
in all seven samples over the entire 28-day hold-
80
ing time, whether samples were acidified to pH 2
or not (Fig. 17). The behavior of 2ADNT and
4ADNT was sample-dependent. For three samples,
the concentrations of these compounds in the acidi-
fied subsamples were substantially lower than for
40
the unacidified subsamples (Fig. 18). In three oth-
Well B
Control
RDX
ers, some at nearly identical initial concentrations
and measured pH, no loss of 2ADNT or 4ADNT
Well
HMX
Acidified
was found due to acidification (Fig. 19). As ob-
I
served previously, when loss occurred, the major
portion occurred rapidly over the first few days.
0
10
20
30
For TNT, acidification to pH 2 proved to be
Storage Time (days)
effective in preserving TNT whether samples ini-
Figure 17. Stability of HMX and RDX in groundwater
tially had TNT present or were fortified with TNT.
samples from the Naval Surface Warfare Center.
For their unacidified counterparts, the results were
mixed: TNT was stable over the 28-day holding
time in two unfortified samples but declined in
portion of sodium bisulfate had been added to
two others. The worst case was for well F (Table
one bottle in each pair so that an acidified and an
A6), where the acidified sample had a mean con-
unacidified subsample from each well were re-
centration of about 22 g/L over the study, but
turned to the laboratory. Samples were shipped
the unacidified sample showed a consistent de-
cold by overnight carrier.
cline from 14 g/L to less than detectable (detec-
Upon receipt in the laboratory the day after
tion limit estimated at 2 g/L). The concentration
sample collection, the pH of all samples was mea-
at 7 days, the currently accepted holding time,
sured and all 36 unacidified samples were screened
was 9 g/L, indicating that nearly two-thirds of
using several commercial enzyme immuno-assay
the TNT had been lost over this period. The con-
kits to estimate the TNT concentration (Thorne and
centration of TNT in the unacidified portion, even
Myers, in press). Based upon the TNT concentra-
in the day 0 sample, was reduced relative to the
tion obtained, nine samples were selected to con-
acidified portion, apparently due to loss occur-
duct holding-time studies, and 40-mL aliquots of
ring during the one-day shipping time from the
both the acidified and unacidified portions of these
field to the laboratory. TNT stability in the forti-
samples were fortified with additional TNT and
fied samples was similarly unpredictable; TNT
TNB. Fortification was accomplished by addition
concentrations in some remained stable but de-
of TNT and TNB in aqueous solution prepared
clined significantly in others (Fig. 20) over the 28-
without use of organic solvents. The pH for each
day holding time.
sample and the fortification level for TNT and
The behavior of TNB in fortified samples paral-
TNB is given in Appendix A. A second set of 40-
leled that of TNT, but the rate of loss appeared to
mL aliquots of each pair of acidified and unacidi-
be faster in samples that showed losses (Fig. 21).
fied samples was also retained without fortifica-
18




 Previous Page
Previous Page
