EM 1110-2-2907
1 October 2003
rived from the subatomic vibrations of matter and is measured in a quantity known as
wavelength. The units of wavelength are traditionally given as micrometers (m) or na-
nometers (nm). Electromagnetic energy travels through space at the speed of light and
can be absorbed and reflected by objects. To understand electromagnetic energy, it is
necessary to discuss the origin of radiation, which is related to the temperature of the
matter from which it is emitted.
c. Temperature. The origin of all energy (electromagnetic energy or radiant energy)
begins with the vibration of subatomic particles called photons (Figure 2-2). All objects
at a temperature above absolute zero vibrate and therefore emit some form of electro-
magnetic energy. Temperature is a measurement of this vibrational energy emitted from
an object. Humans are sensitive to the thermal aspects of temperature; the higher the
temperature is the greater is the sensation of heat. A "hot" object emits relatively large
amounts of energy. Conversely, a "cold" object emits relatively little energy.
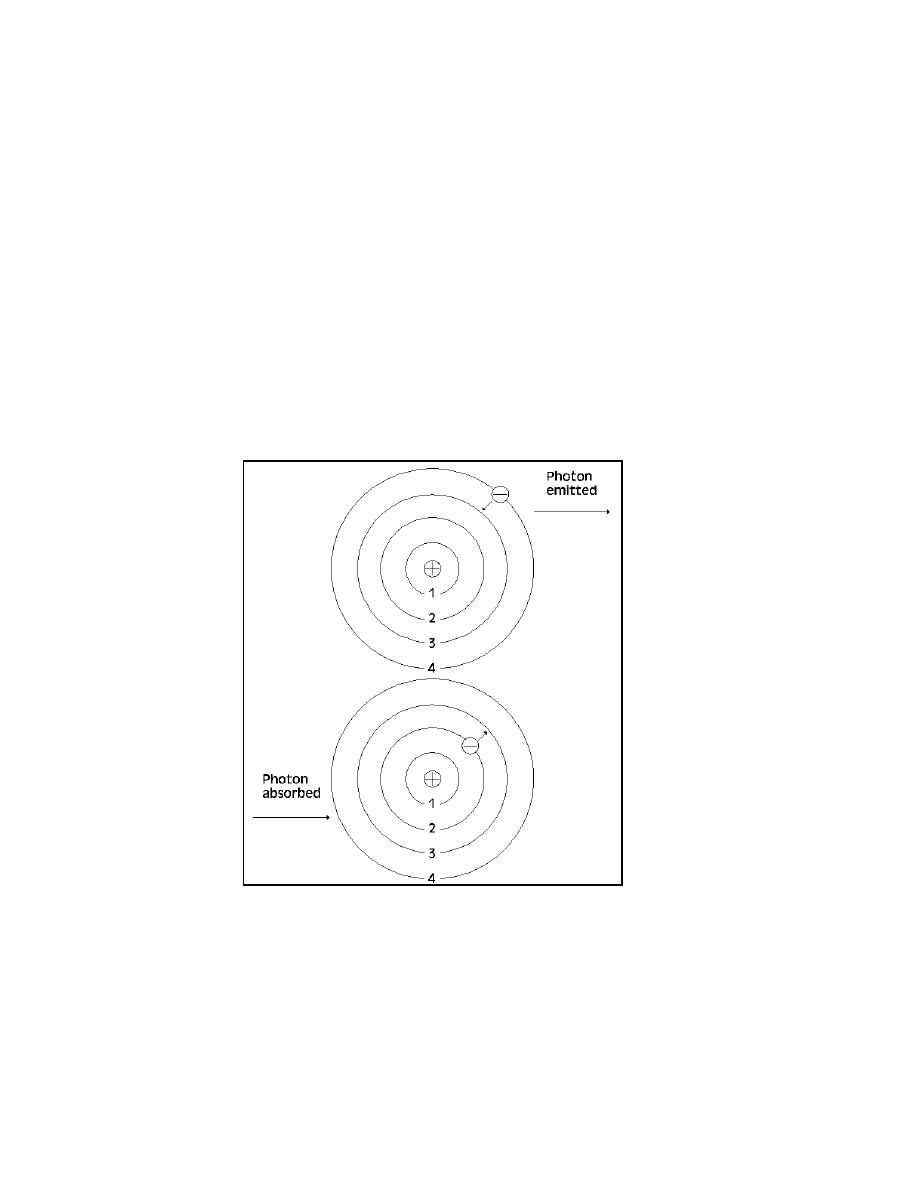
Figure 2-2. As an electron jumps from a higher to
lower energy level, shown in top figure, a photon of
energy is released. The absorption of photon energy
by an atom allows electrons to jump from a lower to
a higher energy state.
d. Absolute Temperature Scale. The lowest possible temperature has been shown to
be 273.2oC and is the basis for the absolute temperature scale. The absolute temperature
scale, known as Kelvin, is adjusted by assigning 273.2oC to 0 K ("zero Kelvin"; no de-
2-3




 Previous Page
Previous Page
