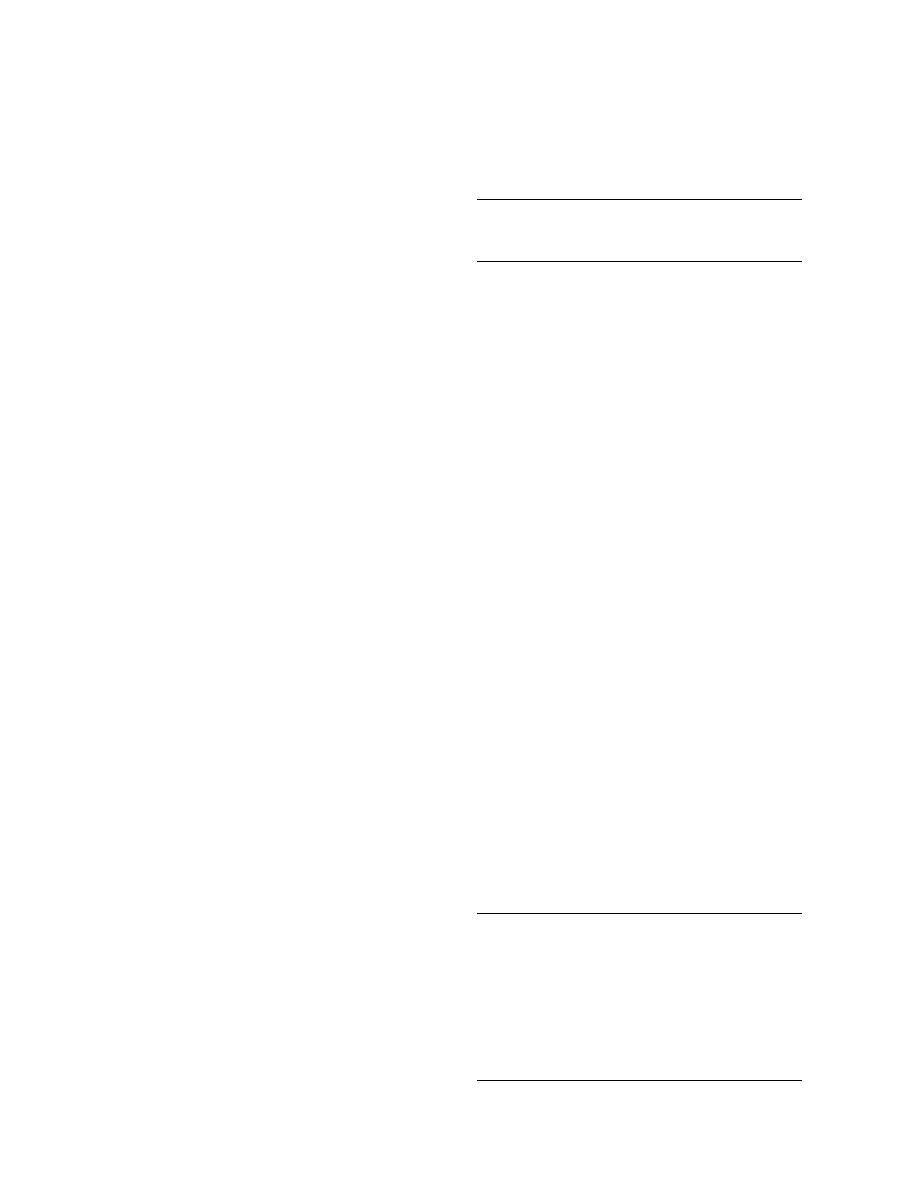
Table 3. Comparison of δ13C and δ15N values for
duce isotopic fractionation. Two methods had to
be developed, one for soil samples and one for
TNT subjected to different sample preparation
water samples. The goal of each method was
processes.
exhaustive extraction with no isotopic fraction-
δ13C
δ15N
ation, because if this occurred during sample
preparation, it would confound the results.
Control solution
28.2
3.41
For soil samples, the most efficient means of
SPE cartridge extract
28.0
3.42
exhaustive extraction is the Soxhlet extractor.
Soxhlet extract
28.2
3.41
Although Soxhlet extractions are slow, this tech-
nique has the unique characteristic that the
analytes are completely separated from the soil in
the δ13C values for TNT, although varied, are not
the extractor because the solvent reservoircollec-
greatly affected by the changing concentration.
tion vessel and sample holder are physically sepa-
The result for the 1.96-g/L (2.0-ng) standard
rated. In the case of ultrasonic extraction, the
shows a dramatic increase in the δ13C value for
sample and extraction solvent are in the same ves-
TNT (Table 4). These data indicate that, below a
sel. Complete (100%) removal of the solvent for
concentration threshold of 4 mg/L, the δ13C value
an ultrasonic extraction is not possible. Some
for TNT takes on a concentration dependency. This
residual solvent remains with the sample and this
dictates that all analyses require an injection mass
solvent will contain some of the analytes. With
of 4 ng or greater.
Soxhlet extraction, once the analytes are passed to
the collection vessel, they cannot again come in
Analysis of TNT from multiple sources
contact with the soil. The results of an extraction
TNT was obtained from several sources, provid-
kinetics experiment showed that a 24-hour extrac-
ing SARM, technical grade, and three military
tion was required to obtain 99.9% recovery of TNT
grade samples. Solutions of each material were
from field contaminated soil using AcN as the
analyzed to determine the δ13C and δ15N values
extraction solvent.
for TNT. The objective of this experiment was to
For extraction of TNT from water samples, solid
establish a working range for these values using
phase extraction (SPE) was the method of choice.
real material that may be present at a TNT-con-
The groundwater was passed through an SPE car-
taminated site. The results are presented in Table
tridge at 10 mL/min and the analytes were recov-
5 and Figure 2.
ered with 5 mL of AcN. The recovery for TNT was
There were significant differences in both the
99.8%.
δ
13C and δ15N values for TNT among the differ-
Spiked soil and water samples were prepared
ent sources. These differences can be attributed to
and processed using the methods described above.
the actual materials used to produce each TNT and
The extracts from these samples were analyzed to
determine the δ13C and δ15N values for TNT. These
the level of refinement for each. The SARM grade
results were compared to the δ13C and δ15N val-
is noticeably the most different. It is also the most
highly refined. For the military grade samples, the
ues for TNT dissolved in solution at the same con-
centration as the extracts. Results for both tech-
niques showed that there was no isotopic
Table 4. Concentration of TNT in solution ver-
sus δ13C value for TNT.
fractionation during the sample preparation pro-
cesses (Table 3)
δ13C value
Concentration
Mass of
of TNT
TNT injected
for TNT
Calibration study
(mg/L)
(ng)
(‰)
The concentration of TNT in moist soil samples
is known to decrease with time under certain
1001
1000
31.9
environmental conditions (Grant et al. 1993). Thus,
501
501
31.2
the mass of TNT recovered over a time course
250
250
28.5
125
125
29.6
experiment will decline as time passes. The objec-
62.5
62.5
27.9
tive of this calibration experiment was to deter-
31.3
31.3
26.2
mine if δ13C values for TNT in solution varied as
15.6
15.6
25.4
a function of concentration or mass injected into
7.8
7.8
29.4
the analyzer. The results in Table 4 show that,
3.9
3.9
34.5
1.96
2.0
17.2
above 4.0 mg/L (or 4.0 ng injected) in solution,
8




 Previous Page
Previous Page
