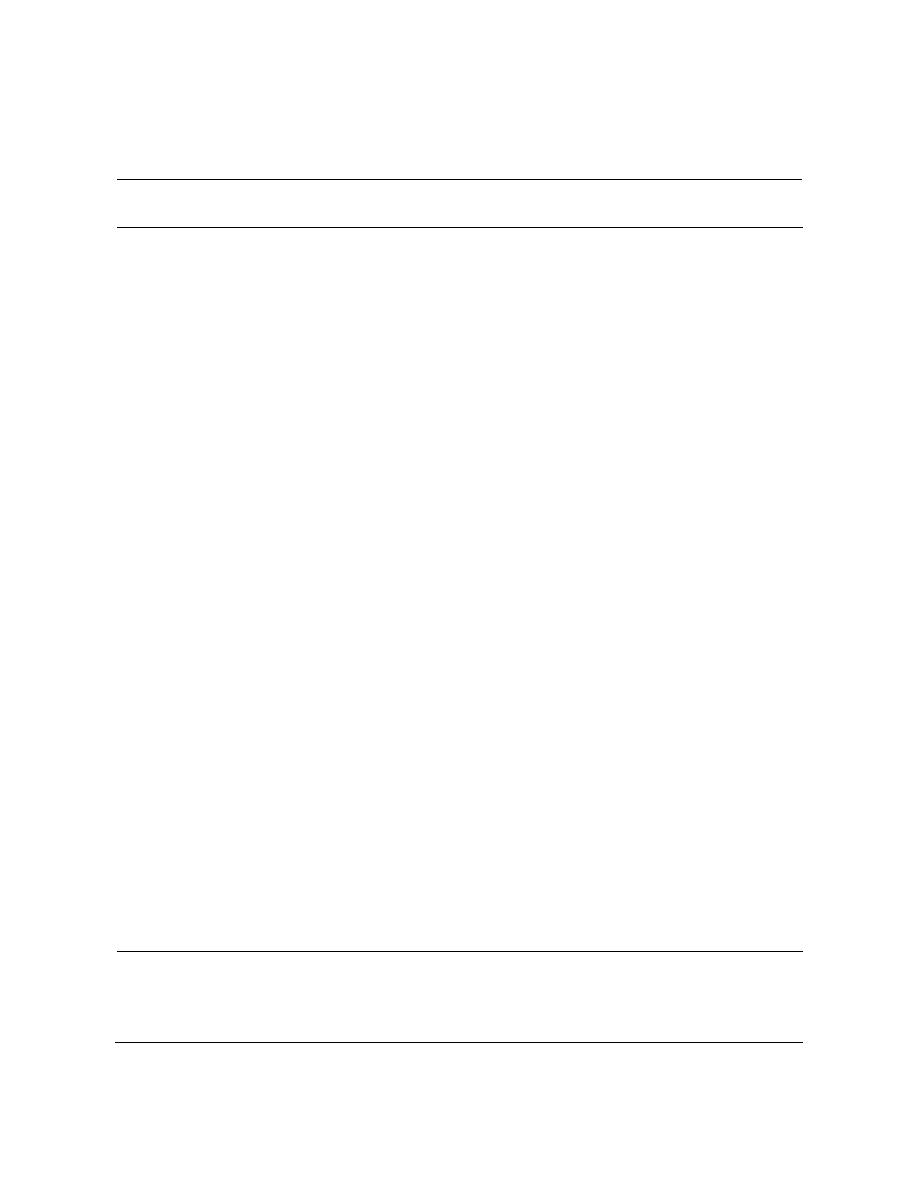
Table 4. Separation of nitramines and nitrate esters with laboratory-grade solvents.
RDX
HMX
PETN
NG
Rf S.D.
Rf S.D.
Rf S.D.
Rf S.D.
Solvent systems
n
0.72 0.01
0.62 0.01
0.91 0.03
0.88 0.02
Petroleum ether : acetone (1 : 1)
3
0.30 0.02
0.84 0.03
0.79 0.02
Petroleum ether : isopropanol (4 : 1)
3
No movement
intensity variations. The fluorescent and
HPTLC plates were evaluated here to determine
nonfluorescent plates were found to produce iden-
if the separation and resolution of compounds
tical separations. However, the plates having
were better relative to standard TLC plates when
preconcentration zones did give better analyte
using conventional techniques. Two different
resolution, compared to plates having no
brands of HPTLC plates were evaluated (EM and
preconcentration zones, when the spotting volume
Adsorbosil). Both brands had preconcentration
exceeded 5 L. This result was in agreement with
zones, with the EM plates also having channeled
Rabel and Palmer (1992) and Hauck and Mack
zones while the Adsorbosil did not. The com-
(1990), in which they report enhanced resolution,
pounds were spotted along the preconcentration
reproducibility, and recovery of analytes spotted
zone using microcapillary dispensers. Because of
the size of the plates (10 10 cm) and thinner thick-
on preconcentration zones.
ness (150200 m), the developing time was usu-
ally between 10 and 15 minutes, half the develop-
Evaluation of HPTLC plates
According to Fenimore and Davis (1981), when
ment time of standard TLC plates. The HPTLC
high-performance thin layer chromatography
plates also required less mobile phase volume
(HPTLC) plates are used in conjunction with mod-
compared to standard TLC plates. However, when
ern scanning equipment, the limit of detection can
the compounds were visualized with UV light or
be similar to those obtained by high-performance
with visualizing agents, the separation and reso-
liquid chromatography (HPLC). HPTLC plates,
lution of compounds, including nitroaromatics,
like conventional TLC plates, are usually coated
nitramines, and nitrate esters, were similar to the
with various binders to hold sorbent material to-
standard TLC plates (Table 6). The two brands of
gether. However, the dimensions of HPTLC plates
HPTLC plates behaved similarly and results
are approximately half the size of conventional
showed no difference in separation and resolution
plates. The particle sizes of the sorbent material
of compounds between plates having channeled
are much smaller and the size distribution of these
zones and no channeled zones.
particles is much tighter. HPTLC plates are also
thinner and the surface is more uniform than con-
Evaluation of visualizing agents
ventional plates. These differences often can re-
Numerous visualizing agents as well as UV light
sult in use of smaller sample volume, smaller sol-
were evaluated for their effectiveness in visualiz-
vent volume for the mobile phase, shorter solvent
ing components of explosives. In most cases, the
migration distance, and greater sensitivity for the
evaluated visualizing agents were chosen in ac-
detection of separated compounds.
cordance with the literature. The summary of the
Table 5. Separation of nitramines and nitrate esters with commercial-brand solvents.
RDX
HMX
PETN
NG
Rf S.D.
Rf S.D.
Rf S.D.
Rf S.D.
Solvent systems
0.58 0.04
0.53 0.04
0.80 0.05
3M adhesive cleaner : Sterling acetone (1 : 1)*
0.78
0.54 0.01
0.45 0.03
0.73 0.05
0.69 0.06
Parks VM & P naphtha: Sterling acetone (1 : 1)*
0.53 0.01
0.42 0.02
0.65 0.06
0.63 0.04
Sterling VM & P naphtha: Sterling acetone (1 : 1)*
0.53 0.03
0.43 0.08
0.59 0.03
0.60 0.03
Sterling Thin-X : Sterling acetone (1 : 1)**
0.48 0.07
0.36 0.09
0.61 0.04
0.59 0.05
Ace paint thinner : Sterling acetone (1 : 1)*
*n = 2
**n = 3
7




 Previous Page
Previous Page
