through filter paper. The detection tubes used for this method have been available as a special order
item from Supelco (Bellefonte, Pennsylvania).
In 1990 we reported a colorimetric-based method for TNT in soil (Jenkins 1990). In this method,
20-g samples of field-moist soils are extracted with 100 mL of acetone by shaking manually for three
minutes (Table 1), allowing the soil to settle, and filtering the extract through a 0.5-m syringe filter.
The resulting extract is then reacted with potassium hydroxide and sodium sulfite to form the high-
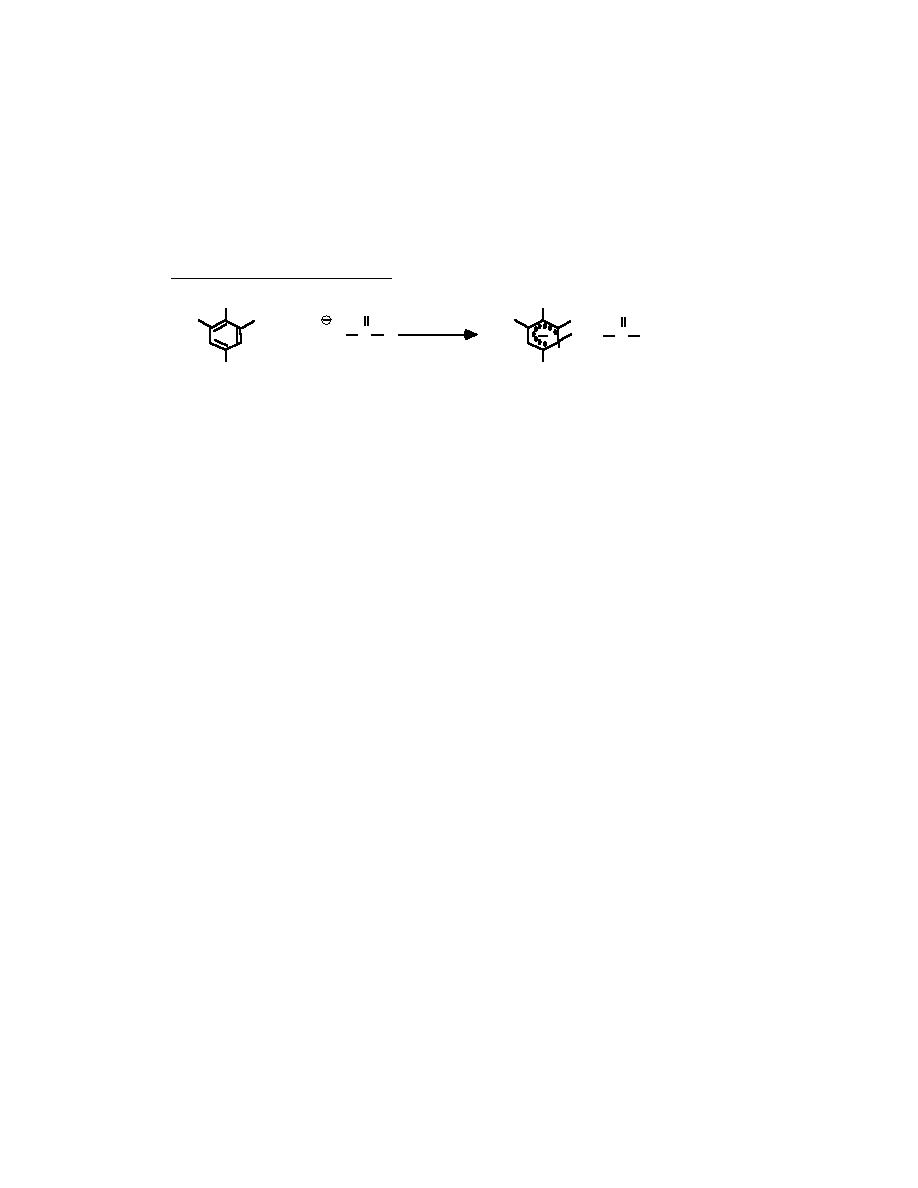
ly colored (reddish for TNT) Janowsky anion (eq 2),
Equation 2. JANOWSKY ANION
CH 3
CH 3
O
O
NO2
O2N
O2N
NO2
+
CH
C
R
CH
C
R
2
2
H
NO2
NO2
TNT
and the TNT concentration is estimated from absorbance measurements at 540 nm. A similar meth-
od was developed by Medary (1992) at about the same time. Medary's method uses a 6-g portion of
field-moist soil and 35 mL of methanol, and requires manual shaking for one minute for extraction
(Table 1). The extracted TNT is reacted with a 10% aqueous solution of sodium hydroxide. TNT is
estimated from the absorbance of the colored Meisenheimer anion produced at 516 nm (eq 1). The
method developed by Medary is sometimes referred to as the Corps of Engineers method.
EnSys (Research Triangle Park, North Carolina) commercialized a colorimetric method for TNT
in soil using an approach similar to that developed by Jenkins (1990). The EnSys method (RisC)
specifies that a 10-g portion of dried soil is extracted with 50 mL of acetone. Extraction is conducted
by manually shaking for three minutes, allowing the soil to settle for five minutes, and filtering the
extract through a 0.5-m Millex SR syringe filter. This method has been issued as SW846 Method
8515, "Colorimetric Screening Method for Trinitrotoluene (TNT) in Soil," by the USEPA Office of
Solid Waste.
Enzyme immunoassay (EIA) methods for TNT
Keuchel et al. (1992a, b) and Keuchel and Neissner (1994) were the first to report the develop-
ment of a competitive enzyme immunoassay (EIA) method to detect TNT in water. The immuno-
assay used polystyrene microtiter plates coated with antibodies. Enzyme-analyte conjugate was
synthesized from 2,4,6-trinitrobenzene sulfonic acid conjugated to the enzyme horseradish peroxi-
dase.
A commercially available enzyme immunoassay for TNT in water and soil was issued in 1993 by
EM Science (Gibbstown, New Jersey) as D TECH Environmental Detection Systems (Hutter et al.
1993, Teaney et al. 1995). This assay makes use of a competitive reaction between enzyme-labeled
TNT and free TNT for binding sites on antibody-coated latex particles. The particles are trapped on
a membrane, washed clean of unbound enzyme conjugate, and treated with a substrate to induce a
color change inversely proportional to the amount of free TNT in the sample. Homogenized, field-
moist soils are collected in a calibrated 3-mL syringe, transferred to a plastic bottle, and extracted
with 6.5 mL of acetone. Extraction is conducted by manually shaking several times over a three-
minute period, allowing the soil to settle for one minute, and pipetting off a 1.0-mL aliquot of the
supernatant. Results are quantitated with a hand-held, dual-beam reflectometer that measures the
difference between the sample and the reference control.
Three other commercial enzyme immunoassay methods are currently available for TNT. The
EnviroGard TNT kit (Millipore ImmunoSystems, Scarborough, Maine) is intended to be a laborato-
ry assay for semi-quantitative analysis of TNT in both soil and water. Field-moist soil samples are
3




 Previous Page
Previous Page
