400
Benzene
100
C
Toluene
D
200
80
B
A
60
0
3
9
15
21
a. Subsample wetted with 3 mL of water acidified with
NaHSO4 and stored at 22C.
Holding Time (days)
40
40
20
20
0
2
4
6
8
10
12
14
Holding Time (days)
0
2
6
10
14
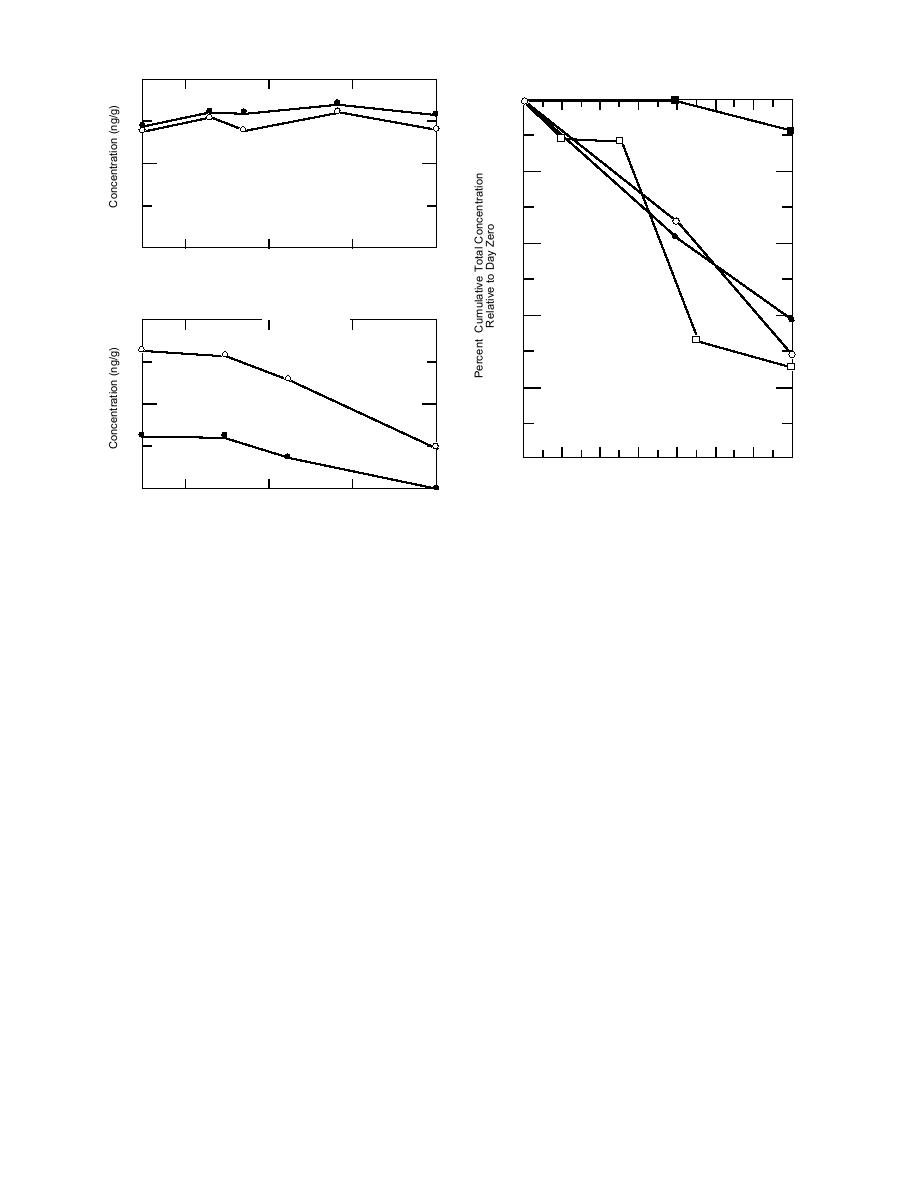
Figure 3. Percent cumulative concentration relative
Holding Time (days)
to day 0 for the treatment level soil subsample sets (A,
b. Subsample moistened with 0.2 mL of water and stored
B, C; Table 3) and for a set (D) handled under similar
at 4C. (From Hewitt 1994c.)
protocols (Hewitt 1994c). All subsamples were prepared
Figure 2. Holding time results for subsamples pre-
for HS/GC analysis and held at room temperature with-
pared for PT/GC/MS analysis.
out chemical preservation.
1994c) for a set of subsamples held under similar
sumption is that the chemical preservatives inhib-
conditions. The only difference between these ex-
ited the activity of the microbes that were indig-
periments was that subsamples were sacrificed up-
enous to the soil. Furthermore, since the soil
on analysis in the treatment level study, where as
subsamples were vapor-fortified just prior to ini-
previously the subsamples had been repeatedly
tiating the experimental conditions, the VOCs were
analyzed. In the absence of chemical preservation,
most likely readily available. For this reason, labo-
the rate of loss due to biodegradation in the CRREL
ratory vapor-fortified soil samples may represent
soil is fairly reproducible. This figure also indicates
a worst-case scenario. There are, however, some
that biodegradation in a given soil can be sup-
precautions that need to be addressed. For in-
pressed when treated with high concentrations of
stance, only four VOCs were tested in this study.
these four VOCs. The cumulative VOC concentra-
Moreover, when acidification is used, it is most
tion where biodegradation appears to be inhibited
likely important that a pH of 2 or lower is obtained
in the CRREL soil is around 240 g/g, well above
and that the soil subsample becomes completely
the treatment level used in the chemical preserva-
dispersed once enclosed in the VOA vial.
tion studies. Therefore,we can be reasonably cer-
Analyte transformations due to the MeOH or
tain that the stability of the Ben and Tol concentra-
NaHSO4 are unlikely. Methanol is commonly used
tions in acidified samples and samples immersed
as the solvent for preparing VOC standards, and a
in MeOH is attributable solely to chemical preser-
study performed with laboratory water, surface
vation.
water and groundwater treated with 25 VOCs and
Even though the success of these two methods
acidified with NaHSO4 showed no chemical in-
of chemical preservation were relative to labora-
terferences (Maskarinec et al. 1990).
tory-fortified samples, field samples most likely
Not included in this report are the results from
will behave similarly. The reasoning for this as-
an experiment using NaHSO4 to acidify subsam-
6




 Previous Page
Previous Page
