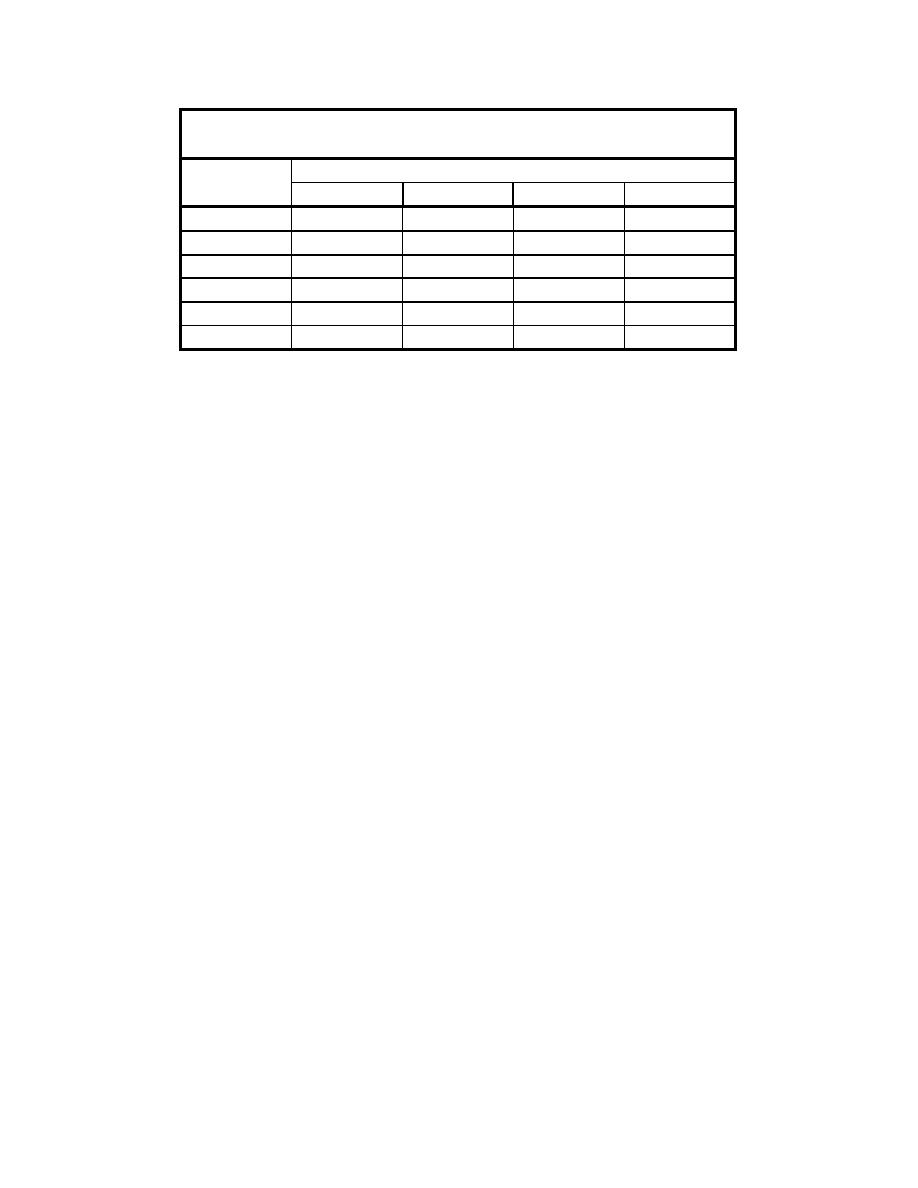
Table 15
High Explosives Investigated in the Dissolution Kinetics Study
High Explosive
TNT
RDX
HMX
Stabilizer
TNT
x
RDX
x
HMX
x
x (39.5%)
x (59.5%)
(trace)
wax (1%)
Octol
x (30%)
x (70%)
LX-14
x (95.5%)
estane (5.5%)
specified time intervals five separate samples were collected from the beaker for
analysis. Water was temperature equilibrated by placement in a water bath that
was insulated from room temperature with a layer of 20-mm hollow plastic balls.
Bath water was circulated through a refrigeration unit to control temperature.
Surface areas were based on study design parameters and the characteristics of
the high explosives available. Energy inputs other than temperature also affect
dissolution rates. Rainfall energy is one source of such energy input. To replicate
this fluctuation in energy input, three differing stirring rates, 90, 150 and
210 rpm, were used.
Surface area. Surface areas of the pure explosives were calculated using
two different methods. For TNT a surface area to mass ratio was determined by
breaking TNT into sets of grains (varied from 5 to 12 grains per set), measuring
length and width of individual grains under a microscope with a calibrated opti-
cal reticle, and then weighing the sets. A good relationship between mass and
estimated surface area could be drawn when grains were longer than about
2.5 mm; therefore, tests were performed with grains longer than 2.4 mm.
The TNT surface area to mass ratio was approximately 23.28 cm2 g-1
(0.04296 g cm-2). Because HMX and RDX crystal sizes were too small and
varied to measure by this technique, an alternate approach was employed. The
military specifications for each explosive are based on standard sieve analyses.
This information, combined with explosive density, assuming the crystals were
spherical, and assuming evenly distributed crystal size distributions, permits an
estimation of surface area. HMX and RDX surface area to mass ratios were
estimated to be 1.12 and 0.10 cm2 mg-1, respectively.
Analytical protocol. Five hundred microliters of each sample was pipetted
into a clean 4-ml vial to which 500 L of 0.45-m filtered acetonitrile was added
and the vial capped. The vial was then vortexed for 5 sec and allowed to stand
for at least 25 min. Samples were pipetted into 0.3-mil glass inserts with a kim
spring held in 4-ml glass vials and sealed with a Teflon/silica cap. Analysis was
performed with a Waters HPLC with a 486 tunable absorbance detector and auto
sampler running Millennium Software package and following Method 8330
(USEPA 1994).
54
Chapter 3 Transport Parameters for Firing Range Residues




 Previous Page
Previous Page
