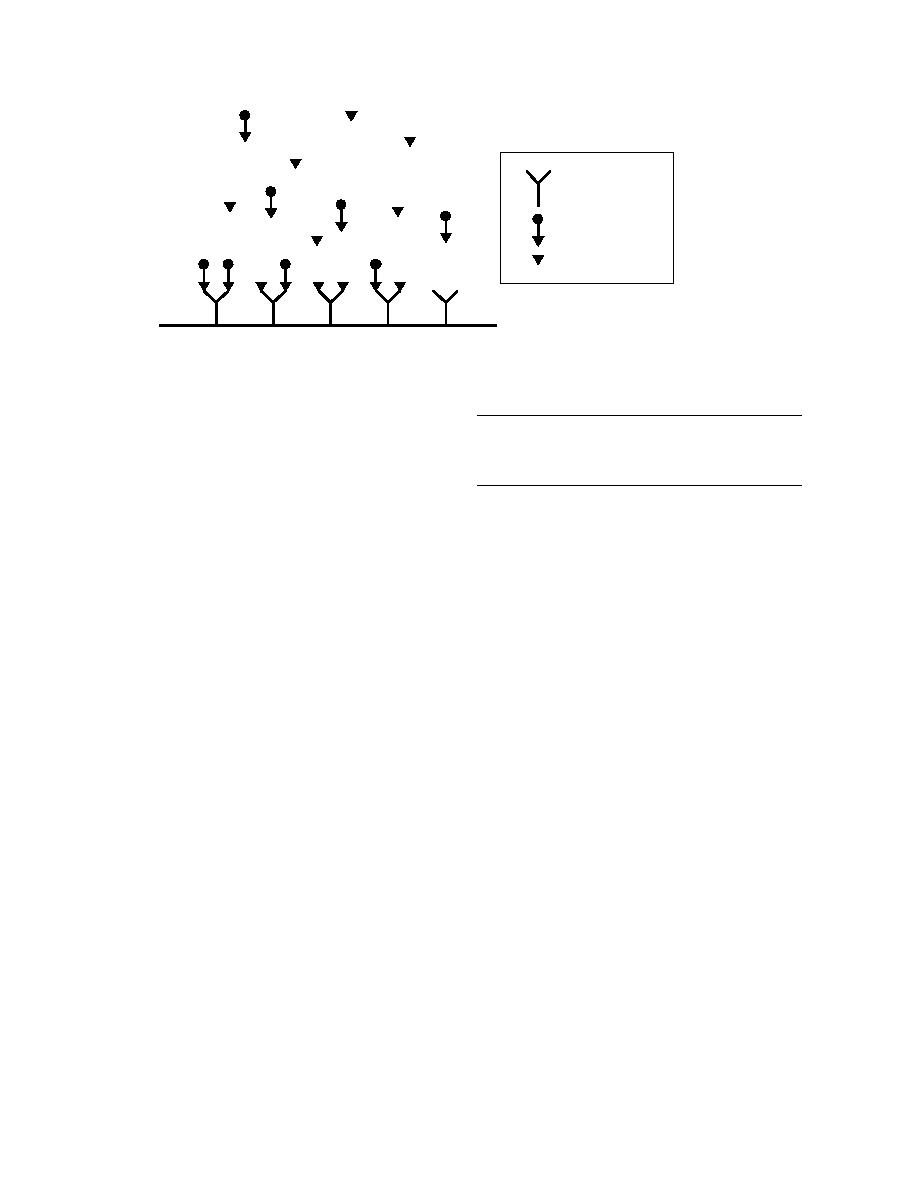
Antibody
Enzyme-Analyte
Conjugate
Free Analyte
Figure 1. Enzyme-linked immunoassay.
Table 1. Cross-reactivities of TNT kits at the 50%
amount of enzyme conjugate retained on the anti-
inhibition (midrange) and detection limit (g/L).
bodies (i.e., color change) is inversely proportional
to the amount of target analyte in the sample
EnviroGard
Ohmicron
DTECH
Quantix
(Vanderlaan et al. 1990). In other words, the more
Analyte
50%/DL
50%/DL
50%/DL
50%/DL
intense the color development, the lower the con-
TNT
3/0.5
1.44/0.07
22/5
1.00/0.05
centration of free analyte in the sample. A less in-
TNB
95/6
2.20/0.04
96/20
0.47/*
tense color indicates higher concentrations of free
2ADNT
>1000/1.6
45/0.25
200/30
<0.05/*
4ADNT
16/0.7
98/0.10
>500/>500 0.02/*
analyte.
Many environmental contaminants are small
*Quantix could not supply the DL cross-reactivities.
molecules that cannot induce antibody production
range of their kits (IC50). However, the nature of
by themselves. These molecules must be covalently
antibodyhapten interaction produces a curve of
bound to larger carrier proteins in order to stimu-
concentration vs. binding that is sigmoidal (Fig. 2).
late antibody production when injected into an
Thus, at concentrations close to the detection lim-
animal. These small moleculeprotein conjugates
its of each kit, the cross reactivities tend to be more
are called haptens. The specificity of an antibody
pronounced. Environmental degradation prod-
to a target analyte can be influenced by the design
of the hapten. This is done by controlling the orien-
ucts of TNT, such as TNB and amino-DNTs, can
tation and spacing between the analyte and carrier
produce a significant additional response.
protein used to induce the immunological effect.
Inaccurate responses in immunoassays can also
Through careful selection of antibodies it is possi-
be caused by compounds that disrupt either anti-
ble to design immunoassays that can distinguish
body binding or enzyme activity. This phenome-
an analyte from a related family of compounds or
non is called interference. In either case, the
a parent compound from its metabolites (Keuchel
amount of color development will be less than
et al. 1992a,b). In the case of TNT, conjugates could
anticipated--i.e., a false positive response. This is
be made by coupling a protein to either a reactive
a better choice than a test that would result in false
moiety at the 1- position ( e.g., trinitro-sulphonic
negatives due to environmental interferences. All
acid) or at the 2- or 4- position (2- or 4-aminodinitro-
of the kits tested reduce potential interferences by
diluting the sample in an assay solution containing
toluene). The antibody would then tend to recog-
a buffer and bovine serum albumin to reduce the
nize either a trinitro-aromatic or a dinitro-toluene,
effects of extreme pH and humic materials (Keuchel
respectively. The binding of antibodies to non-
et al. 1992c).
target analytes is termed cross reactivity. For mole-
DTECH produces the only immunoassay for
cules with limited numbers of functional groups,
specificity becomes more difficult and cross reac-
detection limit. It has no response to nitroaromat-
tivity with other structurally related molecules be-
comes more likely.
ics.
The various schemes that were used by the four
manufacturers to produce anti-TNT antibodies re-
EXPERIMENTAL METHODS
sulted in a wide variety of cross-reactive analytes
and relative degrees of interference (Table 1). In
Collection of groundwater samples
general, manufacturers emphasize the degree of
Groundwater samples were collected from 33
cross-reactivity that occurs in the middle of the
monitoring wells at the Naval Surface Warfare
2




 Previous Page
Previous Page
