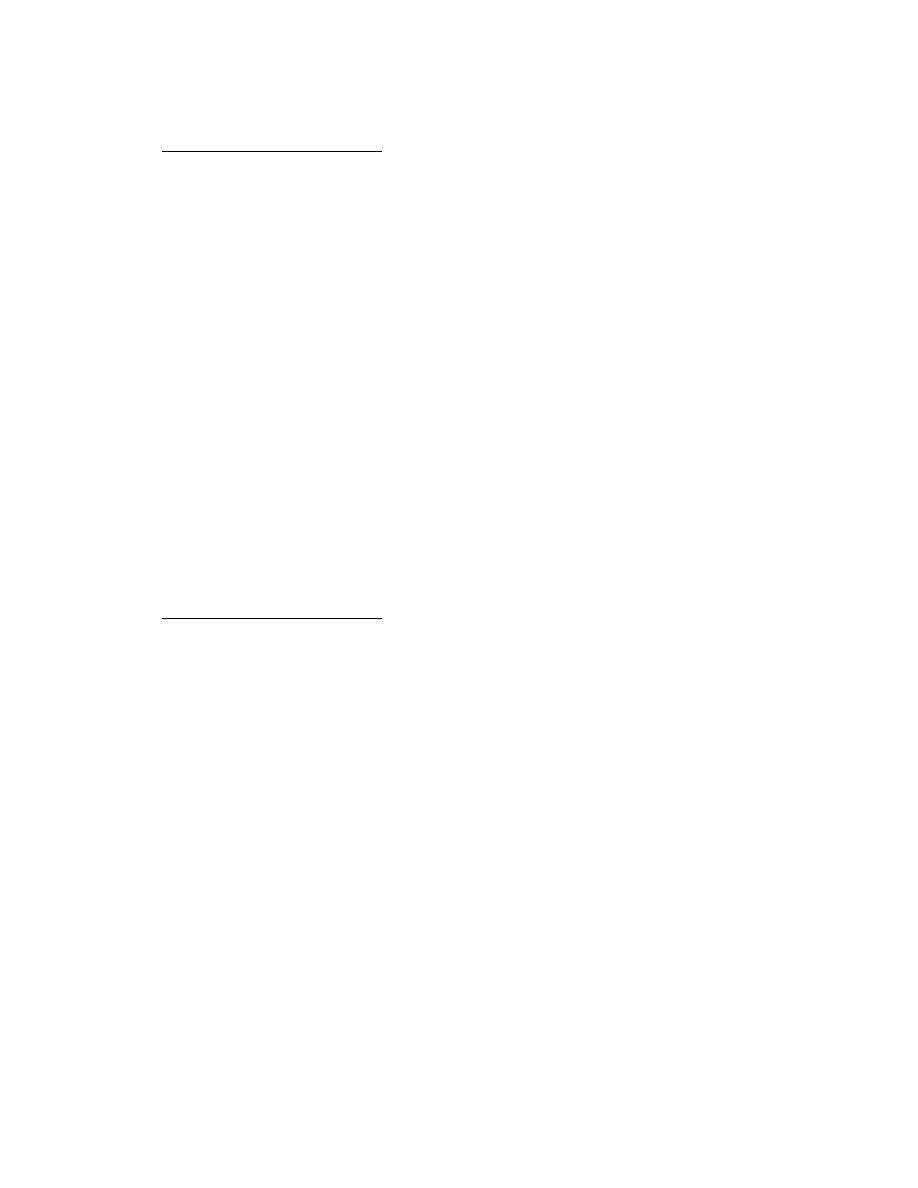
Table 2. Estimates of the Flory
al. 1992) was that the method used to determine
Huggins interaction parameter χ.*
softening (i.e., manually bending the sample) was
(After Vonk 1985.)
subjective and lacked quantification.
As reported in a subsequent paper (Parker and
χ
Organic compound
Ranney 1994), we conducted several studies (short
Benzene
0.83
term and long term) using a Barcol Impressor to
Toluene
0.82
quantitate hardness. In our short-term studies,
o-xylene
0.76
small test pieces of PVC were exposed to various
m-xylene
0.87
activity (0.8, 0.6, 0.4, 0.0) solutions of methylene
p-xylene
0.86
chloride for a week. The pieces exposed to the
Ethylbenzene
0.89
1,3,5-trimethylbenzene
2.52
0.6- and 0.8-activity solutions had hardness read-
Propylbenzene
1.91
ings that were significantly lower than the con-
Methylethylketone
0.54
trol samples, and these samples were rubbery in
Di-isopropylketone
0.56
that they could easily be bent. This was not the
Methylene chloride
0.55
case for the samples exposed to the 0.4-activity
1,2-dichloroethane
0.56
1,1,1-trichloroethane
0.92
solution. Thus, we concluded that samples turned
1,1,2-trichloroethane
0.58
rubbery at a lower activity (0.6) than would be
1,1,2,2-tetrachloroethane
0.59
predicted using Berens' isotherms, unless the true
1,2-dichloropropane
0.65
χ value for methylene chloride is approximately
Perchloroethylene
1.58
0.3 or less.
Trichloroethylene
0.90
Chlorobenzene
0.66
In a long-term study (Parker and Ranney 1994),
1,2-dichlorobenzene
0.63
PVC was exposed to lower activity solutions of
1,3-dichlorobenzene
0.74
methylene chloride (0.4, 0.2, 0.1, 0.05, 0.0) for 20
1,2,4-trichlorobenzene
1.52
weeks. By the end of the study, there were sig-
o-chlorotoluene
0.74
nificant changes in the hardness readings for
m-chlorotoluene
0.69
p-chlorotoluene
0.75
samples exposed to 0.4-, 0.2- and 0.1-activity so-
Nitrobenzene
0.54
lutions but not to the 0.05-activity solutions.
0.87
However, none of the samples with the lower
2-chloroaniline
0.58
hardness readings were actually rubbery or pli-
3-chloroaniline
0.61
able. We concluded that softening had begun to
n-methylaniline
0.59
occur at much lower activities than had been pre-
* For segments of PVC pipe (O.D. = 32
viously reported or predicted by either Berens or
mm and wall thickness = 1.6 mm) im-
Vonk and that softening might occur at even lower
mersed in pure liquid compounds for 410
activities (< 0.1) if given more time. However, we
days at 20 3C.
also thought that it might be possible that only
partial softening occurs at these lower activities
and that the samples never actually become rub-
tions. Also as Berens predicted, samples exposed
bery or pliable. We concluded that a longer-term
to the lowest-activity solution (0.2) showed no
study is necessary to resolve this issue, and we
signs of softening by the end of the study. How-
are currently conducting such a study.
ever, samples exposed to the 0.4- and 0.6-activity
We also tested the ability of a second organic
solutions showed some slight changes (i.e., occa-
solvent, trichloroethylene (TCE), to soften PVC
sionally a sample could be bent and/or showed
(Parker and Ranney 1994). TCE is one of the most
signs of curling), especially at the 0.6 activity.
common organic pollutants in the environment
According to Berens, PVC should not be softened
and is also a relatively good swelling agent of
by any organic solvent at an activity of 0.4, even
PVC [χ = 0.88 (Berens 1985) or χ = 0.90 (Vonk 1985)].
if χ were equal to 0.0. Using Vonk's value for χ
In our first study with TCE, pieces of PVC were
(0.55) and Berens's isotherms (Fig. 1), we would
exposed to various activity (1.0, 0.8, 0.6, 0.4, 0.2,
also predict that softening should not occur at an
0.0) solutions for up to 68 days. By the end of the
activity of 0.6. However, if Berens is correct and
χ is less than 0.53 and if the true value is 0.3 (or
experiment, samples exposed to the 0.6-, 0.8- and
less), then softening would be expected (at 30C).
1.0-activity solutions had Barcol readings that were
significantly lower than the controls, although
Thus, it appears that softening is occurring at
none of these samples appeared to be plasticized
slightly lower activities than would be expected.
or rubbery.
However, the problem with this study (Parker et
3



 Previous Page
Previous Page
